

Author byline as per print journal: Brian Godman1,2,3, BSc, PhD; Eleonora Allocati4, BSc, MSc; Evelien Moorkens5, MSc, PhD; Hye-Young Kwon, PhD1,6
|
Abstract: |
The recent GaBI Journal manuscript by Bertolani and Jommi comprehensively assessed the implications of a range of policies including education, benchmarking and financial incentives, implemented by the different healthcare organisations (HCOs) among the regions in Italy to increase the use of biosimilars as a way to conserve resources. This included both prospective and retrospective analyses of shifts in prescribing behaviour among the different regions and potential savings generated as well as how the savings generated were used [1].
We linked the findings of the Bertolani and Jommi paper [1] with other recent studies in an attempt to stimulate ongoing debate regarding potential ways to enhance the future use of biosimilars as well as how best to utilize the considerable resource savings produced [2, 3] without compromising care.
The Bertolani and Jommi study is seen as particularly important as a source of information that can be used to provide future guidance as there have only been a limited number of studies to date that assessed differences in regional policies to enhance the prescribing of biosimilars in the ambulatory care setting where biologicals are increasingly being used [4]. A response rate of 38% to the survey is seen as acceptable [5, 6], especially since the regions surveyed covered 93% of the Italian population [1].
The need to leverage competition from biosimilars will only increase since without a major increase in the use of biosimilars global expenditure on medicines is projected to reach US$1.5 trillion by 2023 [7–9]. This growth will be primarily driven by increased expenditures on specialty biological medicines, including new medicines for chronic, complex, or rare diseases, such as cancer and orphan diseases. Global expenditure for these medicines is likely to reach 50% of total medicine expenditures in the near future [7, 10]. Such expenditures are difficult to sustain, especially in countries with universal healthcare systems that need to fund a growth in the use of medicines driven by increasing rates of non-communicable diseases, changes in clinical practice, and the continual launch of new, high-priced medicines that address areas of unmet need [11–14]. There are additional concerns raised about some new, high-priced medicines for cancer and orphan diseases, for which funding appears driven more by the emotive nature of these diseases than by their proven clinical benefits [15–18]. Their value is being increasingly questioned as more medicines advocated in guidelines become available as low cost, multi-sourced medicines or biosimilars [19–21].
Biological medicines under increasing scrutiny include Humira® with global sales of US$19.9 billion in 2018. Although Humira® sales are now being decreased, especially in Europe, through increasing use of lower priced biosimilars as well as by the fact that AbbVie lowered the price of Humira® to compete [22–26]. For example, among Danish hospitals, expenditures for adalimumab decreased by 82.8% following the availability of biosimilars with almost total replacement by biosimilars (95.1% utilization). In the UK, expenditure on adalimumab is envisaged to fall by 75% following the availability of biosimilars [23, 25]. Such reductions are welcomed, especially among lower- and middle-income countries, including Central and Eastern European countries, where the use of biologicals has been limited by available governmental resources as well as by high patient co-payments [27–29]. Biosimilar switching programmes have been shown to conserve resources by a number of studies that also were unable to demonstrate meaningful differences in effectiveness or safety between biosimilars and originators. Such studies have included infliximab and other biologicals across a range of indications [30–38]. There have, however, been some concerns requiring patient monitoring [39, 40]. Such concerns could be exacerbated by multiple modifications in the manufacturing of originator biological drugs that can occur without companies being required to undertake clinical studies to assess the effect of such changes on clinical outcomes in actual practice, even with major manufacturing changes [41, 42].
Other biological medicines of special interest to health authorities across Europe and beyond include rituximab, infliximab and etanercept with current global sales of US$7.9 billion, US$5.9 billion and US$ 5.8 billion in 2017, respectively [43–45]. These concerns persist despite the fact that these sales are being reduced as the result of the increasing use of lower cost biosimilars [38, 39, 45-49]. Global sales of Herceptin® (trastuzumab) were stable in 2019 at US$7 billion due to increasing use of biosimilar trastuzumab. The use of biosimilar trastuzumab is expected to continue to increase globally. Trastuzumab biosimilars have already captured 45% of the European market [50–52]. However, these savings are being offset by growing expenditures on pertuzumab in combination with trastuzumab; with annual sales of US$2.8 billion in 2019 as well as by the use of Kadcyla® (trastuzumab emtansine), which is expected to reach annual sales of US$4.94 billion by 2023 [50, 53].
Another important biological is long acting insulin glargine used for patients with Type 1 diabetes. The insulin glargine market was valued at approximately US$3.88 billion in 2018; and is envisaged to reach US$9.26 billion by 2025 [54]. However, potential savings associated with the introduction of biosimilar insulin glargine have been hampered by the limited price reductions seen in practice in a number of countries. There are also concerns with switching because differences in devices between manufacturers could increase the rate of hypoglycaemia [55–58]. These concerns have resulted in some health authorities advising against switching, despite similar effectiveness and safety being demonstrated in studies comparing the originator and a biosimilar [55, 56, 59-62]. The situation is however changing, at least in the US where a biosimilar insulin glargine reached over 40% of market share in the US Medicaid programme in 2018. There are also a number of initiatives and publications pushing for increased use of biosimilars among European countries including those encouraging new patients to be started on a biosimilar [49, 63-66].
A number of published studies have shown the potential for considerable savings from biosimilars. These results are pushing health authorities to employ initiatives to enhance their use [3, 9, 38, 49, 51, 67-70]. Winegarden (2019) in the US estimated annual savings of up to US$7 billion from the use of a range of biosimilars, and that these savings are likely to grow as more biosimilars become available [3]. However, for maximum savings, both supply- and demand-side measures are needed [21, 69]. This has been shown in studies of oral generic medicines comparing potential policies and savings in Korea with those in the UK [71–74]. Multiple demand- and supply-side measures in Scotland resulted in a considerable reduction in expenditure on lipid lowering medicines and proton pump inhibitors despite appreciably increased volumes [73, 74]. Moorkens et al. (2017), Rémuzat et al. (2017) and Vogler et al. (2017) have all recently summarized ongoing demand-side measures in Europe to enhance the use of biosimilars. Simoens et al. (2018) also provided guidance on additional demand-side measures that could be introduced to further realize the benefits of biosimilars [75–78]. There are European countries where some lower-cost biosimilars now account for the total market, e.g. erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF) [79], and countries such as France and the UK are actively working to increase biosimilar penetration rates [80, 81].
Brill in the US has recently discussed the benefits of shared savings to enhance biosimilar use among State Medicaid programmes [10]. Siu et al. (2019) documented ongoing activities to enhance the use of biosimilars in both the private and public sectors in Canada [70]. These activities include preferential coverage by private insurers for increasing use of biosimilars, the pan-Canadian Oncology Biosimilars Initiative to enhance adoption of biosimilars in oncology (an attempt to address concerns with funding in oncology), as well as the British Columbia Biosimilars Initiative in May 2019 that promotes switching, with the savings used to lower premiums and co-pays where pertinent [70]. In addition, Biosimilars Canada has recently developed a centralized patient support service platform to assist manufacturers and patients with increasing the use of biosimilars [70]. Such activities are needed to promote the use of biosimilars because originator manufacturers have been appreciably lowering prices of their originators just before patent expiry to dissuade biosimilar companies from entering the market [22, 44, 79]. Suggestions have been made that originator companies should automatically lower their prices following patent expiry, thereby negating the need for biosimilars to interfere with the market in the first place [82]. Methods are needed to counter other behaviours of originator companies such as developing new formulations just before patent expiry to create a barrier to biosimilars mirroring other evergreening tactics [79, 83].
It was impressive to see that 89% of HCO surveyed by Bertolani and Jommi had implemented policies to enhance the use of biosimilars [1]. Educational activities were particularly prominent, increasing in recent years, including information on market access pathways for biosimilars as well as the results of tenders. Educational activities are crucial to allay fears regarding the effectiveness and safety of biosimilars. These fears are illustrated in Italy by the seven scientific Italian societies that recently expressed concerns about the Regional Administrative Court of Piemonte promoting the automatic substitution of biologicals in terms of therapeutic continuity for patients and concerns with the freedom of prescribing clinicians [84]. Despite this, benchmarking of biosimilar prescribing among physicians was already taking place among 75% of HCOs surveyed and this is likely to grow since such benchmarking of physician prescribing is working well in other countries [1, 85]. The 62% of HCOs that also provided physicians with prescribing targets for biosimilars is similar to what has been seen in other countries and regions [86–88], with 68% and 24% respectively, introducing incentives and sanctions to improve prescribing rates. Sanctions include monetary sanctions and potentially removal of the right to prescribe [1].
A concern though is that patients were involved in educational/information programmes among only 22% of the HCOs surveyed [1]. This is a potentially important weakness since all key stakeholders need to be convinced about the value of biosimilars in order to reduce any potential nocebo effects [89, 90].
The study of Bertolani and Jommi adds to a number of examples of successful multiple demand-side measures including preferentially encouraging the prescribing of multi-sourced medicines versus originators and patented medicines in a class without compromising care [91–93]. Monies saved can subsequently be used to fund new more expensive medicines as well as other healthcare services such as diagnostics. Ninety-three per cent of HCOs also provided physicians with information retrospectively or prospectively on potential savings from increased use of biosimilars, with 25% of HCOs also participating in post-marketing studies to help further fears with biosimilars [1]. However, only 21% of HCOs systematically estimated the proportion of potential patients not receiving biosimilars, with only rare perceptual surveys among patients and other healthcare professionals. This situation may need to change given the stated concerns of the seven scientific Italian societies [84].
In conclusion, Bertolani and Jommi, have provided a comprehensive review of ongoing policies among HCOs in the different regions of Italy and their potential to influence on future directions. This information is useful for other countries where demand-side measures can be localised to meet future goals, with the potential for localities to learn from each other. This is important to stimulate increasing use of biosimilars in a way that addresses the accelerating resource challenges brought about by the expanding use of medicines in ageing populations as well as the need to pay for new, high-priced medicines that address areas of previously poorly or untreatable diseases.
There was no funding for this paper.
Competing interests: The authors have no conflicts of interest to declare.
Provenance and peer review: Commissioned; internally peer reviewed.
Brian Godman1,2,3, BSc, PhD
Eleonora Allocati4, BSc, MSc
Evelien Moorkens5, MSc, PhD
Hye-Young Kwon, PhD1,6
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK
2Division of Public Health Pharmacy and Management, School of Pharmacy, Faculty of Health Sciences, Sefako Makgatho Health Sciences University, Pretoria, South Africa
3Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
4Istituto di Ricerche Farmacologiche ‘Mario Negri’ IRCCS, Milan, Italy
5KU Leuven Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium
6College of Pharmacy, Seoul National University, Seoul, Korea
References
1. Bertolani A, Jommi C. Local policies on biosimilars: are they designed to optimize use of freed resources? Generics and Biosimilars Journal (GaBI Journal). 2020;9(4):163-70. doi:10.5639/gabij.2020.0904.027
2. Pettit C. Biosimilars market is ripe for cost savings. Am J Manag Care. 2019.
3. Winegarden W. The biosimilar opportunity: a state breakdown. Pacific Research Institute Publication. 2019.
4. Moorkens E, Simoens S, Troein P, Declerck P, Vulto AG, Huys I. Different policy measures and practices between Swedish counties influence market dynamics: Part 2-Biosimilar and originator etanercept in the outpatient setting. Biodrugs: 2019;33(3):299-306.
5. Pandya C. What is an acceptable response rate for online surveys? AppJetty. 2019.
6. Lindemann N. What’s the average survey response rate? [2019 benchmark]. 2019. Available from: https://surveyanyplace.com/average-survey-response-rate/
7. IQVIA. The global use of medicine in 2019 and outlook to 2023. Forecasts and areas to watch. 2019 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023
8. Inotai A, Csanádi M, Vitezic D, Francetic I, Tesar T, et al. Policy practices to maximise social benefit from biosimilars. J Bioequiv Availab. 2017;9:467-72.
9. Povero M, Pradelli L. Funding innovation thanks to anti-TNF-a biosimilars uptake: the economic impact in Italy. Farmeconomia. Health economics and therapeutic pathways. 2020;21(1):37-47.
10. Brill A. Shared savings demonstration for biosimilars in medicare: an opportunity to promote biologic drug competition. 2020. Matrix Global Advisors.
11. Morgan SG, Bathula HS, Moon S. Pricing of pharmaceuticals is becoming a major challenge for health systems. BMJ. 2020;368:l4627-l.
12. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
13. Gyasi RM, Phillips DR. Aging and the rising burden of noncommunicable diseases in Sub-Saharan Africa and other low- and middle-income countries: a call for holistic action. Gerontologist. 2020;60(5):806-11.
14. Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, et al. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
15. Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
16. Luzzatto L, Hyry HI, Schieppati A, Costa E, Simoens S, Schaefer F, et al. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392(10149):791-4.
17. Godman B, Wild C, Haycox A. Patent expiry and costs for anticancer medicines for clinical use. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(3):105-6. doi:10.5639/gabij.2017.0603.021
18. Haycox A. Why cancer? Pharmacoeconomics. 2016;34(7):625-7.
19. Godman B, Hill A, Simoens S, Kurdi A, Gulbinovič J, Martin AP, et al. Pricing of oral generic cancer medicines in 25 European countries; findings and implications. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(2):49-70. doi:10.5639/gabij.2019.0802.007
20. Derbyshire M, Shina S. Patent expiry dates for biologicals: 2017 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(1):29-34. doi:10.5639/gabij.2019.0801.003
21. Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34(2):159-70.
22. Sagonowsky E. AbbVie’s massive Humira discounts are stifling Netherlands biosimilars: report. Fierce Pharma. 2019 Apr 2.
23. Jensen TB, Kim SC, Jimenez-Solem E, Bartels D, Christensen HR, Andersen JT. Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern Med. 2020;180(6):902-3.
24. Stanton D. Global Humira sales near $20bn, but expect decline in 2019. BioProcess International. 2019 Jan 28.
25. Davio K. After biosimilar deals, UK spending on adalimumab will drop by 75%. Center for Biosimilars. 2018 Nov 26.
26. Davio K. Researchers predict substantial savings for Europe on the strength of biosimilar adalimumab. Center for Biosimilars. 2019 May 21.
27. Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279.
28. Kostic M, Djakovic L, Šujić R, Godman B, Jankovic SM. Inflammatory bowel diseases (Crohn’s disease and ulcerative colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85-93.
29. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198-206.
30. Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304-16.
31. Høivik ML, Buer LCT, Cvancarova M, Warren DJ, Bolstad N, Moum BA, et al. Switching from originator to biosimilar infliximab – real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53(6):692-9.
32. Milassin Á, Fábián A, Molnár T. Switching from infliximab to biosimilar in inflammatory bowel disease: overview of the literature and perspective. Therap Adv Gastroenterol. 2019;12:1756284819842748.
33. Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463-78.
34. Gisondi P, Bianchi L, Calzavara-Pinton P, Conti A, Chiricozzi A, Fimiani M, et al. Etanercept biosimilar SB4 in the treatment of chronic plaque psoriasis: data from the Psobiosimilars registry. Br J Dermatol. 2019;180(2):409-10.
35. Stebbing J, Baranau YV, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, et al. Double-blind, randomized phase III study to compare the efficacy and safety of CT-P6, trastuzumab biosimilar candidate versus trastuzumab as neoadjuvant treatment in HER2 positive early breast cancer (EBC). J Clin Oncol. 2017;35(15_suppl):510.
36. Pegram MD, Bondarenko I, Zorzetto MMC, Hingmire S, Iwase H, Krivorotko PV, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer. 2019;120(2):172-82.
37. Tweehuysen L, Huiskes VJB, van den Bemt BJF, Vriezekolk JE, Teerenstra S, van den Hoogen FHJ, et al. Open-label, non-mandatory transitioning from originator etanercept to biosimilar SB4: six-month results from a controlled cohort study. Arthritis Rheumatol. 2018;70(9):1408-18.
38. Chan A, Kitchen J, Scott A, Pollock D, Marshall R, Herdman L. Implementing and delivering a successful biosimilar switch programme – the Berkshire West experience. Future Healthc J. 2019;6(2):143-5.
39. Nisar MK. 292 Switching to biosimilar rituximab: a real world study. Rheumatology. 2018;57(suppl_3).
40. Ratnakumaran R, To N, Gracie DJ, Selinger CP, O’Connor A, Clark T, et al. Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand J Gastroenterol. 2018;53(6):700-7.
41. Jiménez-Pichardo L, Gázquez-Perez R, Sierra-Sánchez JF. Degree of prescriber’s knowledge about variability in biological drugs “innovators” in manufacturing process. Eur J Clin Pharmacol. 2018;74(4):505-11.
42. Vezér B, Buzás Z, Sebeszta M, Zrubka Z. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin. 2016;32(5):829-34.
43. Global tumor necrosis factor inhibitors drug market, dosage, price & clinical pipeline outlook 2024. PRNewswire. 2018 Mar 16.
44. Hoen E’t. Humiragate: AbbVie’s desperate attempts to keep its monopoly. Medicines Law & Policy. 2019.
45. Davio K. Roche’s European rituximab sales drop 11% due to biosimilar competition. Center for Biosimilars. 2018 Feb 5.
46. Davio K. Biosimilar competition leads to 9.7% drop in Remicade sales for Johnson & Johnson. Center for Biosimilars. 2018 Jan 23.
47. Matusewicz W, Godman B, Pedersen HB, Fürst J, Gulbinovič J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Exp Rev Pharmacoecon Outcomes Res. 2015;15(5):755-8.
48. Småstuen MC, Madla R, Solli O, Hjelvin E. OP0311 retrospective analysis of prescription dynamics of etanercept after introduction of biosimilars based on Norwegian prescription database. An interim analysis. Ann Rheumatic Dis. 2019;78:237-8.
49. Mansell K, Bhimji H, Eurich D, Mansell H. Potential cost-savings from the use of the biosimilars filgrastim, infliximab and insulin glargine in Canada: a retrospective analysis. BMC Health Serv Res. 2019;19(1):827.
50. Taylor P. Roche’s Herceptin/Perjeta fixed-dose combo filed with FDA. PMLive. 2020 Feb 25.
51. Lee SM, Jung JH, Suh D, Jung YS, Yoo SL, Kim DW, et al. Budget impact of switching to biosimilar trastuzumab (CT-P6) for the treatment of breast cancer and gastric cancer in 28 European countries. BioDrugs. 2019;33(4):423-36.
52. Jeremias S. Biosimilars gain market share and advocates get aboard. Center for Biosimilars. 2020 Aug 15.
53. GlobalData. PharmaPoint. Kadcyla (HER2-positive breast cancer) – forecast and market analysis to 2023. 2014 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.marketresearch.com/product/sample-8464560.pdf
54. Zion Market Research. Insulin glargine market: by type (pre-filled syringe and single dose vial), by application (type 1 diabetes and type 2 diabetes), by distribution channel (hospital pharmacy, online sales, retail pharmacy and other distribution channels): global industry perspective, comprehensive analysis and forecast, 2018–2025. 2019 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.zionmarketresearch.com/report/insulin-glargin-market
55. NHS Lothian. Lothian Formulary. 6.1.1 Insulins. 2020 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.ljf.scot.nhs.uk/LothianJointFormularies/Adult/6.0/6.1/6.1.1/Pages/default.aspx
56. Greater Glasgow and Clyde. Medicines Update – prescribing medicines by brand. 2020 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: http://www.ggcprescribing.org.uk/blog/prescribing-medicines-brand/
57. Aladul MI, Fitzpatrick RW, Chapman SR. Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: a qualitative study. BMJ Open. 2018;8(11):e023603.
58. Greener M. Why isn’t the NHS making the most of biosimilar insulin? Prescriber August 2019: 21-4.
59. Heinemann L, Carter AW. Will biosimilar insulins be cheaper? Diabetes Technol Ther. 2017;19(9):513-5.
60. Yamada T, Kamata R, Ishinohachi K, Shojima N, Ananiadou S, Nom H, et al. Biosimilar vs originator insulins: systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(7):1787-92.
61. Blevins TC, Barve A, Raiter Y, Aubonnet P, Athalye S, Sun B, et al. Efficacy and safety of MYL-1501D versus insulin glargine in people with type 1 diabetes mellitus: results of the INSTRIDE 3 phase 3 switch study. Diabetes Obes Metab. 2020;22(3):365-72.
62. Lamb YN, Syed YY. LY2963016 insulin glargine: a review in type 1 and 2 diabetes. BioDrugs. 2018;32(1):91-8.
63. Greater Glasgow and Clyde. Medicines Update – Semglee® – preferred brand of insulin glargine. 2020 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: http://ggcprescribing.org.uk/blog/alternatives-insulin-glargine-post-tc/
64. Saborido-Cansino C, Santos-Ramos B, Carmona-Saucedo C, Rodríguez-Romero MV, González-Martín A, Palma-Amaro A, et al. [Effectiveness of an intervention strategy in the biosimilar glargine prescription pattern in primary care]. Aten Primaria. 2019;51(6):350-8.
65. Agirrezabal I, Sánchez-Iriso E, Mandar K, Cabasés JM. Real-world budget impact of the adoption of insulin glargine biosimilars in primary care in England (2015-2018). Diabetes Care. 2020;43(8):1767-73.
66. Hernandez I, Good CB, Shrank WH, Gellad WF. Trends in Medicaid prices, market share, and spending on long-acting insulins, 2006-2018. Jama. 2019;321(16):1627-9.
67. González-Fernández M, Villamañán E, Jiménez-Nácher I, Moreno F, Plasencia C, Gaya F, et al. Cost evolution of biological agents for the treatment of spondyloarthritis in a tertiary hospital: influential factors in price. Int J Clin Pharm. 2018;40(6):1528-38.
68. Gulácsi L, Brodszky V, Baji P, Rencz F, Péntek M. The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther. 2017;34(5):1128-44.
69. Kim Y, Kwon HY, Godman B, Moorkens E, Simoens S, Bae S. Uptake of biosimilar infliximab in the UK, France, Japan, and Korea: budget savings or market expansion across countries? Front Pharmacol. 2020;11:970.
70. Siu ECK, Tomalin A, West K, Anderson S, Wyatt G. An ever-evolving landscape: an update on the rapidly changing regulation and reimbursement of biosimilars in Canada. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(3):107-18. doi:10.5639/gabij.2019.0803.014
71. Kwon HY, Godman B. Drug pricing in South Korea. Appl Health Econ Health Policy. 2017;15(4):447-53.
72. Kwon HY, Kim H, Godman B, Reich MR. The impact of South Korea’s new drug-pricing policy on market competition among off-patent drugs. Expert Rev Pharmacoecon Outcomes Res. 2015;15(6):1007-14.
73. Leporowski A, Godman B, Kurdi A, MacBride-Stewart S, Ryan M, Hurding S, et al. Ongoing activities to optimize the quality and efficiency of lipid-lowering agents in the Scottish national health service: influence and implications. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):655-66.
74. Godman B, Kurdi A, McCabe H, MacBride-Stewart S, Leporowski A, Hurding S et al. Ongoing activities to influence the prescribing of proton pump inhibitors within the Scottish National Health Service: their effect and implications. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(4):142-51. doi:10.5639/gabij.2018.0704.030
75. Simoens S, Le Pen C, Boone N, Breedveld N, Llombart-Cussac A, Jorgensen F, et al. How to realize the potential of off-patent biologicals and biosimilars in Europe? Guidance to policymakers. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(2):70-4. doi:10.5639/gabij.2018.0702.014
76. Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PloS One. 2017;12(12):e0190147.
77. Vogler S, Schneider P. Do pricing and usage-enhancing policies differ between biosimilars and generics? Findings from an international survey. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(2):79-88. doi:10.5639/gabij.2017.0602.015
78. Rémuzat C, Kapuśniak A, Caban A, Ionescu D, Radière G, Mendoza C, et al. Supply-side and demand-side policies for biosimilars: an overview in 10 European member states. J Mark Access Health Policy. 2017;5(1):1307315.
79. European Commission. IQVIA Report 2018: the impact of biosimilar competition in Europe [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://ec.europa.eu/docsroom/documents/31642
80. GaBI Online – Generics and Biosimilars Initiative. Quotas. France aims to reach 80% biosimilar penetration by 2022 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Sep 28]. Available from: www.gabionline.net/Policies-Legislation/France-aims-to-reach-80-biosimilar-penetration-by-2022
81. NHS England. Commissioning framework for biological medicines (including biosimilar medicines). 2017 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf
82. Atteberry P, Bach PB, Ohn JA, Trusheim M. Biologics are natural monopolies (Part 1): why biosimilars do not create effective competition. Health Affairs Blog. 2019. Available from: https://www.healthaffairs.org/do/10.1377/hblog20190405.396631/full/
83. Vernaz N, Haller G, Girardin F, Huttner B, Combescure C, Dayer P, et al. Patented drug extension strategies on healthcare spending: a cost-evaluation analysis. PLoS Med. 2013;10(6):e1001460.
84. Quotidiano Sanita. Oggetto: Sentenza 465/20 TAR Piemonte Sezione I sull’approvvigionamento di farmaci biologici. 14 September 2020 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: http://www.quotidianosanita.it/allegati/allegato628568.pdf
85. Gustafsson LL, Wettermark B, Godman B, Andersen-Karlsson E, Bergman U, Hasselstrom J, et al. The ‘wise list’ – a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108(4):224-33.
86. Godman B, Allocati E, Moorkens E. Ever-changing landscape of biosimilars in Canada; findings and implications from a global perspective. Generics and Biosimilars Initiatives (GaBI Journal). 2019;8(3):93-7. doi:10.5639/gabij.2019.0803.012
87. NHS Scotland. Secondary care national therapeutic indicators 2018/19 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: https://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/08/Secondary-Care-National-Therapeutic-Indicators-Version-1.0.pdf.
88. All Wales Medicine Strategy Group. National prescribing indicators 2018–2019. 2018 [homepage on the Internet]. [cited 2020 Sep 28]. Available from: http://www.awmsg.org/docs/awmsg/medman/National%20Prescribing%20Indicators%202018-2019.pdf.
89. Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10:1372.
90. Kristensen LE, Alten R, Puig L, Philipp S, Kvien TK, Mangues MA, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs. 2018;32(5):397-404.
91. Godman B, Wettermark B, Bishop I, Burkhardt T, Fürst J, Garuoliene K, et al. European payer initiatives to reduce prescribing costs through use of generics.Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
92. Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
93. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):707-22.
|
Author for correspondence: Brian Godman, BSc, PhD; Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, 161 Cathedral Street, Glasgow G4 0RE, UK; Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2020 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/can-local-policies-on-biosimilars-optimize-the-use-of-freed-resources-experiences-from-italy.html
Author byline as per print journal: Brian Godman, BSc, PhD; Claudia Wild, PhD; Alan Haycox, PhD
|
Abstract: |
Submitted: 15 March 2017; Revised: 15 March 2017; Accepted: 17 March 2017; Published online first: 31 March 2017
Drs Brian Godman, Claudia Wild and Alan Haycox review the paper by Venkatesan S et al. on the patent expiry of (non-)tyrosine kinase inhibitors (TKIs) [1].
Venkatesan S et al. are to be congratulated on publishing their interesting paper providing general insight into exclusivity and patent rights for the non-TKIs [1] used in the treatment of patients with cancer. The authors point out that the TKIs and some of the non-TKIs have long exclusive rights, which is a concern especially given some of their marginal or small health gains versus current standards. It is not clear why the patent lives are so long, and why there are such differences between Europe and the US. This may well be because of orphan status and other considerations; however, this information is not provided in their paper. The authors suggest that the long patent life may be due to the limited development time for these compounds; but this may also not necessarily be the case. In any event, as Venkatesan S et al. point out, there is increasing concern with the growing cost of new medicines [2], which would be enhanced by granting premium prices and long patent lives for new medicines. Countries, even high-income countries, are now struggling to fund all new valued medicines, which is not in the interest of any key stakeholder group [2, 3].
There has been a rise in the clinical approvals of kinase inhibitors. Consequently, this is one of the reasons why TKIs were singled out for special attention in this paper. However, the review suggests that all TKIs are equally beneficial, which is not the case. Having said this, imatinib is a concern to payers with sales enhanced by off-label use with initially high prices granted on the basis of orphan status [4]. Global sales were estimated at US$4.75 billion in 2014, making imatinib the 14th highest selling product worldwide that year [5]. The rationale for choosing the non-TKIs is also not fully explained. Nevertheless, the paper gives very good insight into their likely generic drug availability, which is crucial for health authorities given the low prices that could be achieved for some of these cancer medicines [6].
In the discussion, the authors make a number of good points regarding high prices for new cancer medicines. This is a key concern across countries, with prices of new cancer medicines rising up to tenfold during the past decade [7, 8]. Prices for new cancer medicines now average US$150,000 or more per year of life gained [9], often with marginal health gain versus current standards [10]. In their recent review, Grössmann and Wild [11] documented that out of 134 new indications approved for cancer medicines since 2009, no data was available for progression-free survival or overall survival in 27%. A positive impact was seen for median overall survival in 55.5%; however, only 16% showed a difference of more than three months [11], which is increasingly seen as a minimum for a new cancer medicine to be seen as an advance [2, 10]. These concerns with ever increasing prices led to calls by US oncologists to pharmaceutical companies to moderate their growth in the future [12, 13]. High prices are also a major concern to lower- and middle-income countries, which currently account for more than 70% of cancer mortality [14]. Increasing prices of new cancer medicines are also threatening the sustainability of universal health care in those countries that provide this given ever-growing prevalence rates for cancer [7, 15]. This is leading to calls that cancer should no longer be singled out for special attention as this has been exploited [16].
It is estimated by some authors that the cost of bringing a new cancer medicine to the market is lower than US$100 million [13], and that prices of generic bortezomib, dasatinib, everolimus and gefitinib could potentially be as low as 1% of the current selling price [6]. This justifies calls for price moderation for new cancer medicines, as well as initiatives to make generics of valued cancer medicines available as early as possible with the cost of cancer medicines now accounting for an ever increasing proportion of the total costs of cancer care [7]. In the meantime, health authorities need to critically rethink how new cancer medicines should be valued, especially given concerns with surrogate markers [2, 10, 17]. Payers and providers also need to increasingly collaborate before product launch to agree on the likely patient populations that will receive the most benefits from the new cancer medicines in order to limit their budget impact [18], and keep to this, as well as seek extensive discounts through risk-sharing arrangements [2, 19].
Overall, the paper by Venkatesan S et al. gives good insight into likely generic drug availability of key cancer medicines, which is crucial for health authorities given the potentially low prices that could be achieved [6]. The paper also highlights the need for increased transparency in relation to development times and patent periods, the need for new cancer medicines to be appraised similarly to all other medicines for pricing and reimbursement evaluations, and not singled out for special status, as well as greater transparency in pricing and reimbursement evaluations. The latter gives increasing concerns with high prices for new cancer medicines coupled with the low cost of goods of some [6, 13]. Finally, the observation that the development time for these (non-)TKIs is rather short should be investigated further through researching the actual timescales for phases I to III trials and earlier of the TKIs.
Competing interests: None.
Provenance and peer review: Commissioned; internally peer reviewed.
Brian Godman1,2,3, BSc, PhD
Claudia Wild4, PhD
Alan Haycox3, PhD
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, G4 0RE Glasgow, UK
2Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
3Health Economics Centre, University of Liverpool Management School, University of Liverpool, Chatham Street, L69 7ZH Liverpool, UK
4Ludwig Boltzmann Institute of Health Technology Assessment, 7/20 Garnisongasse, AT-1090 Vienna, Austria
References
1. Venkatesan S, Lamfers M, Leenstra S, Vulto AG. Overview of the patent expiry of (non-)tyrosine kinase inhibitors approved for clinical use in the EU and the US. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(2):89-96. doi:10.5639/gabij.2017.0602.016
2. European Parliament. Godman B, Oortwijn W, de Waure C, Mosca I, Puggina A, Specchia ML, et al. Links between pharmaceutical R&D models and access to affordable medicines. A study for the ENVI Committee [homepage on the Internet]. [cited 2017 Mar 15]. Available from: http://www.europarl.europa.eu/RegData/etudes/STUD/2016/587321/IPOL_STU(2016)587321_EN.pdf
3. Malmström RE, Godman BB, Diogene E, Baumgärtel C, Bennie M, Bishop I, et al. Dabigatran – a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;4:39.
4. Kesselheim AS, Myers JA, Solomon DH, Winkel-mayer WC, Levin R, Avorn J. The prevalence and cost of unapproved uses of top-selling orphan drugs. PLoS One. 2012;7(2):e31894.
5. Top 50 pharmaceutical products by global sales. PMLiVE.
6. Hill A, Redd C, Gotham D, Erbacher I, Meldrum J, Harada R. Estimated generic prices of cancer medicines deemed cost-ineffective in England: a cost estimation analysis. BMJ Open. 2017;7(1):e011965.
7. Kelly RJ, Smith TJ. Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. 2014;15(3):e112-8.
8. Bach PB. Reforming the payment system for medical oncology. JAMA. 2013;310(3):261-2.
9. Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139-62.
10. Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium–the just price. J Clin Oncol. 2013;31(28):3600-4.
11. Grössmann N, Wild C. Between January 2009 and April 2016, 134 novel anticancer therapies were approved: what is the level of knowledge concerning the clinical benefit at the time of approval? ESMO Open. 2017;1:e000125. doi:10.1136/esmoopen-2016-000125
12. Tefferi A, Kantarjian H, Rajkumar SV, Baker LH, Abkowitz JL, Adamson JW, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90(8):996-1000.
13. Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439-42.
14. World Health Organization. Hoen Et. Access to cancer treatment. A study of medicine pricing issues with recommendations for improving access to cancer medication [homepage on the Internet]. [cited 2017 Mar 15]. Available from: http://apps.who.int/medicinedocs/documents/s21758en/s21758en.pdf
15. Ghinea N, Kerridge I, Lipworth W. If we don’t talk about value, cancer drugs will become terminal for health systems. The Conversation. 26 July 2015.
16. Haycox A. Why cancer? Pharmacoeconomics. 2016;34(7):625-7.
17. Wild C, Grössmann N, Bonanno PV, Bucsics A, Furst J, Garuoliene K, et al. Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 2016;27(11):2134-6.
18. Matusewicz W, Godman B, Pedersen HB, Fürst J, Gulbinovic J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):755-8.
19. Ferrario A, Kanavos P. Managed entry agreements for pharmaceuticals: the European experience. EMiNet, Brussels, Belgium. LSE Research Online. 2013.
|
Author for correspondence: Brian Godman, BSc, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, G4 0RE Glasgow, UK; Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2017 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/patent-expiry-and-costs-for-anticancer-medicines-for-clinical-use.html
Author byline as per print journal: Babar Khan, BPharm, MPH, PhD; Brian Godman, BSc, PhD; Ayesha Babar, BPharm, MPhil; Shahzad Hussain, BPharm, MPhil; Sidra Mahmood, PharmD, MSc; Tahir Aqeel, BPharm, MPhil
|
Introduction: There are concerns over the quality of generic medicines in Pakistan. This is due to perceived non-compliance with good manufacturing practice (GMP), whereby the quality of the raw materials is not being assessed. If not addressed, this will impact on the potential for generics exports from Pakistan, as well as on patient care. Consequently, there is a need to assess the current assessment and regulatory situation in Pakistan and to recommend a way forward that ensures the future quality of products. |
Submitted: 7 July 2016; Revised: 8 November 2016; Accepted: 14 November 2016; Published online first: 25 November 2016
The pharmaceutical market in Pakistan was worth approximately US$2.3 billion in 2014 [1]. At present, Pakistan produces a variety of medicines and meets approximately 90% of the demand for domestic finished products. However, currently Pakistan only produces a limited amount of the active pharmaceutical ingredients (APIs) needed for medicines consumed in Pakistan [2], with more than 90% of raw materials/APIs coming from China and India.
There are concerns over the quality of medicines manufactured in Pakistan due to perceived non-compliance with good manufacturing practice (GMP) requirements outlined by pharmaceutical manufacturers [3]. These include checks on the quality of APIs being used to produce oral tablets [3]. GMP requirements are included in the Drug (Licensing, Registering and Advertising) Rules (Schedule-B II), which were enacted in Pakistan in 1976 [4]. However, since then, GMP and other registration requirements have not been updated [5]. This means that there have been no updates or revisions following the creation of international standards or World Health Organization (WHO) guidelines [6]. With over 1,200 registered medicines and over 80,000 registered drug products in Pakistan [5], coupled with physician concerns over the safety and efficacy of lower cost generics, the country sees high levels of prescribing of originator products [5, 7, 8]. This is a public health concern as self-pay for medicines in Pakistan is widespread [9] and therefore there are implications for affordability and subsequent adherence rates, especially for treatment of chronic diseases affecting the lower paid [10–12]. Patient care is not compromised with generic medicines that meet agreed quality standards, including bioequivalence levels, across a wide range of disease areas [13–19]. Concerns over generic immunosuppressants are also reducing [20].
There is inconsistency between the information to be included in application dossiers required for authorizing a medicine in Pakistan by the Drugs (Licensing, Registering and Advertising) Rules, 1976, and those stated in the Drug Regulatory Authority of Pakistan (DRAP) Act, 2012 [21]. In this Act, Schedule-I states that the pharmaceutical dossier should include a set of the following documents for submission that give all information on the technical aspects of a product’s manufacture:
a. Master formula
b. All ingredients both active pharmaceutical ingredients and inactive excipients added with their safety profile data
c. Complete manufacturing procedure of the drug, biological or medical device
d. Quality control steps and procedures at each level of raw material selection, in-process testing, finished drug testing and stability testing
e. Clinical trial data and published reports about the safety and efficacy of the drug
f. Complete details of manufacturing plant and equipment, quality control laboratories and equipment
g. Warehouse capacities and facilities; details of human resources available and the latest cGMP (current good manufacturing practice) report shall also be part of this document set
h. Any other information required by the Registration Board for establishing the safety, efficacy, bioavailability, bioequivalence, or biosimilarity of the drug.
Section 7 (c) (ix) of the DRAP Act, also emphasizes the systematic implementation of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), WHO and US Food and Drug Administration (FDA) guidelines [3, 6, 22]. All of these guidelines suggest that application for the registration of medicines in a country should follow an internationally harmonized format known as the Common Technical Document (CTD). The CTD consists of five modules [23]:
However, despite many developing countries implementing such standards, these standards are not implemented in Pakistan. The lack of implementing such standards demonstrates the continued weaknesses of this registration process [5].
The quality of medicines in Pakistan is of major concern. DRAP is currently unable to effectively check the quality of all APIs and finished products through the available surveillance testing laboratories that are under governmental control. These materials are typically only tested to be quantified, and not for the identification of impurities, nor do they undergo any other pharmacopoeia tests. In addition, most of the laboratories involved in conducting these tests appear to contain out-dated instruments and materials, and there are concerns over levels of staff training and availability, as only a limited number of staff have been hired in recent years. This is despite such regulations being included in current laws [24].
The medicine registration process in any country is key to the availability of medicines that meet agreed quality targets. Concerns over the quality of generics produced in Pakistan is currently resulting in low exports [25] and impacting on patient care, especially for patients with chronic diseases.
There are also concerns that the basic and semi-basic industry involved in the manufacture of raw materials in Pakistan has not flourished due to unfavourable policies towards the protection or security of businesses. This is reflected by the fact that only 33 manufacturing units are currently involved in basic or semi-basic manufacturing of pharmaceuticals in Pakistan [26]. This compares with approximately 600 active licensed manufacturers of finished products in Pakistan [27]. In addition, among these 33, only seven manufacturing units currently appear active further demonstrating problems with current policies [26].
Considering these concerns, this study has assessed the quality of APIs in Pakistan and seeks to use the findings as a starting point for suggesting improvements in the registration process for oral generic tablets. We chose ibuprofen for our study in view of the extent to which it is prescribed in Pakistan, which is 12% by value of the analgesic market. The analgesic market currently has a growth of 20% per annum [28]. In addition, APIs of ibuprofen are produced both locally and imported. For ibuprofen, there are specific concerns relating to potential impurities in the light of pharmacopoeia specifications. The findings will subsequently serve as a guide to suggest improvements for the pharmaceutical drug registration process in Pakistan, to ensure good quality, safe, effective and affordable medicines are being produced that will help improve patient care in Pakistan, and potentially boost exports.
Collection of ibuprofen API samples
Twenty-seven samples of ibuprofen APIs used by manufacturers in Pakistan were obtained, together with their Certificate of Analysis (CoA). The CoA contained details regarding the results of tests and their values and limits (including assay values) of relevant batches, as well as information about the manufacturer. The US Pharmacopeia (USP) reference standard ibuprofen was obtained from Abbott Laboratories, Pakistan (originator manufacturer of ibuprofen). Coding was undertaken on all collected samples, with all samples stored in closed containers. Desiccators were used to avoid moisture absorption.
Quality assessment
The quality assessment of the ibuprofen samples was performed using the following methods:
1. Identification test
All samples were identified using the following two methods:
For all API samples and the USP Reference Standard (Abbott Laboratories, Pakistan), the solutions were prepared in 0.1 N Sodium Hydroxide with a concentration of 0.025 g per 100 mL, equivalent to 250 μg per mL of ibuprofen. Respective absorptivities at 264 nm and 273 nm on the anhydrous basis were noted.
For the UV-Spectrophotometer limits, the absorptivities were calculated on an anhydrous basis, and should not differ by more than 3.0% at 264 nm and 273 nm, as per USP limits.
The testing of ibuprofen against USP specifications was undertaken in one of Pakistan’s ‘Appellate Laboratory’, known to perform to the highest standards. The utilization of an USP approved laboratory to undertake the testing has significance in terms of the reliability of the results, negating the need to test the samples in other USP international laboratories in either Brazil or China or India.
2. Assay test
USP specifications mention the following limits for assay testing, ‘Ibuprofen (Active Pharmaceutical Ingredient) contains not less than 97.0 per cent and not more than 103.0 per cent of C13H18O2, calculated on the anhydrous basis’ [29]. The USP only documents one related or impurity substance, this is in contrary to the British Pharmacopoeia specifications where 18 substances are mentioned. However, the identification and characterization of related or impurity substances is out of the scope of this paper.
The Assay Test procedure
Mobile phase
Chloroacetic acid (4.0 g) was dissolved in water (400 mL) and then adjusted to a pH of 3.0 with ammonium hydroxide. Acetonitrile (600 mL) was added, then filtered, and degassed. Amendment or modifications were done as per the requirements of System Suitability.
Preparation of standard solution
A solution with concentration of about 12 mg per mL was prepared by dissolving USP Ibuprofen RS (accurately weighed) in Internal Standard Solution.
Assay preparation
1200 mg of ibuprofen, accurately weighed, was transferred to a 100 mL volumetric flask, diluted with Internal Standard Solution to volume, and mixed. Table 1 contains details of the chromatographic conditions.
The L1 column packing used was Octadecysilane C18 as C18 is generally more retentive than C8.
Test for system suitability
The peak response was recorded after repeated injections of five to six consecutive standard solutions before injecting the sample solutions. This was repeated after completion of work, to observe the consistency of performance of the system.
Acceptability criteria
The qualification criteria for the system suitability test was that there should be less than 2% Relative Standard Deviation (RSD) for five replicate injections of the standard solution, with not more than 2.5 tailing factors for the individual peaks.
Procedure
Equal volumes (approximately 5 μL) of the Standard preparation and the Assay preparation were separately injected into the chromatograph. Chromatograms were recorded and the response for the major peaks was measured.
The quantity of ibuprofen in mg was calculated using the formula: 100 C (RU/RS) where:
1. Identification test
Through FTIR (Fourier Transform Infrared) Spectroscopy:
Table 2 documents the percentage resemblance of the FTIR spectra of the 27 samples, to the USP standard spectrum. It also shows the percentage difference between the 27 samples and the USP standard spectrum, for the 264 nm and 273 nm peaks, recorded via UV-Spectrophotometry.
For measurements taken on the same day, precision ranged from 0.23% to 0.62%, and accuracy ranged from 99.6% to 100.3%. For measurements taken on different days, precision ranged from 0.24% to 0.52%.
2. Assay values
Table 3 documents the assay values of the 27 samples and the ibuprofen USP reference standard.
The results reveal both positive and concerning aspects surrounding the APIs currently used in the production of generic ibuprofen tablets in Pakistan.
All samples, except sample IBU-14, passed the Identification test through FTIR and UV methods as per the requirements of USP specifications, see Table 2. Using the analytical methodology or assessment suggests that 96% API samples passed the test and can be approved and marketed as ibuprofen.
However, 22 out of 27 (81.5%) of the ibuprofen samples failed to comply with the USP assay limits, i.e. 97% to 103%. Interestingly, sample IBU-14 passed this test with highest percentage of assay value, i.e. 99.15%, see Table 3. IBU-14 also showed minimal difference in assay value to the values mentioned in the product CoA, see Table 3.
Secondly, one extra peak was noticed in the chromatograms of six (18.5%) of the samples, i.e. IBU-3, IBU-4, IBU-5, IBU-10, IBU-19 and IBU-25, at approximately the same time, i.e. between 3.1 to 3.3 minutes.
Thirdly, the comparison of assay values obtained using our methodology, versus those claimed by the manufacturer in their CoA, see Table 3, shows that none of the samples complied with the assay values claimed in their CoAs. Instead, three (11%) of the samples showed more than a 5% difference in assay value. In the majority of cases, the manufacturers of the finished products did not perform any testing on the API supplied. Instead, they typically rely on the CoA. This should be of concern to both regulators and manufacturers. Regulators in terms of the implementation of cGMP, while manufacturers should ethically and legally be responsible to follow cGMP for their finished products. In cases where product manufacturers perform tests on the APIs, when assay values of API are found to be lower than the prescribed limits, instead of rejecting the raw material or API, they typically use higher quantities in the production of ibuprofen tablets based on their own calculations, which raises concerns over the quality of products (Hussein S 2016, personal communication, November 8).
Our findings show a general failure of the current system of drug regulation in Pakistan that surrounds the quality of APIs used for drug production. This is in line with previous publications [5]. This will negatively impact on the quality of finished generic products for use in patients and will potentially compromise patient care. The results also show that quality assessment of multiple source medicines should not rely on assay testing alone. Other pharmacopoeia tests are also important, especially in cases where the optical activity and the presence of genotoxic, and other impurities, have a critical impact or role regarding the efficacy, safety and quality of medicines. Here, the failing sample of ibuprofen (IBU-14) passed the assay test with the highest value, while the sample failed the identification test. This invites scientific discussion regarding the value of current assay testing for generics in Pakistan, see Table 2. The results suggest that IBU-14 was not a pure API of ibuprofen. Instead, it may contain related substances or impurities which have a very close structural resemblance to the API of ibuprofen, and the HPLC method used could not identify these or discriminate them from the actual API of the drug [30]. This is why the USP does not mention the use of the HPLC method for the identification testing of ibuprofen API. Consequently, the USP compendium of methods for the identification of ibuprofen API, i.e. the FTIR and UV-Spectrophotometer methods, should be used in the future to assess the content of APIs in Pakistan.
The extra peak in the chromatograms, at 3.1 to 3.23 minutes, is also an important observation. When samples IBU-3, IBU-4, IBU-5, IBU-10, IBU-19 and IBU-25 (18.5%) were investigated for their source of manufacturing, it was found these samples were procured from only three sources. Two were in Pakistan (B and H) and one was in India (C). Further appraisal revealed that all APIs purchased from sources C and H showed this extra peak at the same time range. This illustrates the necessity to perform prequalification studies to evaluate the quality of the API from pharmaceutical manufacturers, before turning the raw materials into finished goods. However, only two out of seven (approximately 29%) API samples purchased from source B showed this extra peak. These results need to be further investigated for the characterization of this peak through evaluation of the route of synthesis or method of manufacturing of the API in order that potential corrective and preventive measures can be suggested for the future. It seems that this extra peak may be due to residual solvent or impurities remaining in the API. However, this needs further investigating before any definitive statements can be made. We are aware that both the British Pharmacopoeia and European Pharmacopoeia include a test for related substances for ibuprofen, with 18 potential impurities listed in the British Pharmacopoeia. However, as mentioned previously, the USP mentions only one specific impurity.
Assessment of the drug registration process and requirements in Pakistan
In view of the findings regarding the assessment of the current procedures concerning the registration of medicines in Pakistan, the technical requirements outlined in Box 1 are a potential way to improve the quality of medicines in Pakistan.
Currently, medicines being registered in Pakistan are not undergoing full evaluation of their safety and quality, especially in terms of their APIs [5]. Some of the suggested requirements, see Box 1, are not currently a mandatory part of the registration of medicines in Pakistan, e.g. the bioequivalence of generic medicines and even submission of the SmPC (Summary of Product Characteristics) and PIL (Patient Information Leaflet). Others, which are covered under the current rules and procedures, are also not being completely fulfilled, such as performing stability studies and validation studies. Ethically and legally, applicants should be bound to fulfill these commitments and thus perform these studies before marketing their medicines. However, in reality very few companies are complying with these commitments and performing such studies before marketing their medicines (S Hussein personal communication). There was a recent incidence of counterfeit medicines at the Punjab Institute of Cardiology which is thought to be a direct result of such deficiencies in the registration process and negligence in following cGMP [31, 32].
Despite these concerns, pharmaceutical manufacturers in Pakistan currently appear reluctant to perform additional tests or provide more comprehensive information about their medicines, during and after registration. This is thought to be due to the potential negative impact this could have on business (S Hussein, personal communication). In fact, the reverse may be true which would lead to improvement in the quality of generics for consumption in Pakistan, and a greater potential for export to other countries.
Here we outline a number of recommendations that should be considered by the Pharmaceutical Evaluation and Registration (PE & R) Division within DRAP, in consultation with Pakistani pharmaceutical companies, to improve the quality of generics produced by domestic manufacturers for use in Pakistan as well as for exportation.
These include a stepwise plan for the implementation of CTDs and new requirements in line with ICH standards, over one to three years, for example:
Alternatively:
Recently, the efforts of DRAP, in collaboration with USP and WHO, to develop the ‘Road Map for Strengthening the Registration System of Pharmaceutical Products and Biologicals for Human/Veterinary Use in Pakistan’, demonstrates a noticeable step towards improving the quality of medicines in Pakistan. We will be monitoring its progress and making additional recommendations if necessary.
Together with this, there is the ongoing process that seeks to convince pharmaceutical companies through discussions, seminars, and dialogue, of the need to adopt these new requirements for drug registration, to improve patient care and the potential for drug exports. The experiences of other countries that have started to accept such registration dossiers on CTD or e-CTD (Electronic Common Technical Document), should also be communicated within Pakistan to enhance acceptance of updated requirements. These countries include: Australia, Canada, China, Croatia, Japan, Saudi Arabia, Singapore, South Africa, Switzerland, the US, and all EU Member States [33]. In addition, comparison with the pharmaceutical manufacturing industry in Jordan whose population is only eight million compared with 201 million in Pakistan. Jordan currently has 16 pharmaceutical units compared with over 600 units in Pakistan [26, 34]. However, their exports are high with over 80% of produced medicines currently being exported over 60 countries [34, 35]. This includes more than US$4.5 billion alone to Saudi Arabia [35]. In contrast, Pakistani manufacturers in 2013 only exported US$1 billion due to concerns with poor quality [25, 36].
The implementation of ICH Standards should also be applicable to already registered medicines. These improvements may be achieved in the same stepwise manner as described above at the time of their application for renewal of registration starting over the coming year.
Other recommendations may include:
Manufacturers and DRAP may consider the following technical aspects whilst undergoing prequalification of API sources:
If these measures are adopted, patients can expect good quality generics in the future. This is important, given the extent to which medicines in Pakistan are self-pay. Without such measures, patient care using generics and trust in the healthcare system will continue to be compromised, and there will be increased potential for adverse drug reactions [37]. Successful steps have already been taken to address concerns over counterfeit medicines in Pakistan, which need to continue [38]. The above considerations should be the next step to further improve the availability of safe, effective and affordable medicines in Pakistan. This is of great importance given the increasing prevalence of chronic illnesses and diseases in this country, where every third person over the age of 40 is vulnerable to a wide range of diseases [39], and 70% of the population lives on less than US$2 per day [40].
The results of this study document the concerns over the current regulations for assessment of the quality of drug raw materials (APIs) in Pakistan. These need to be urgently addressed to ensure good quality generics in Pakistan for patients, and to improve potential export opportunities. The adoption of WHO and ICH recommended CTD format and WHO prequalification guidelines, to improve the process of registration of medicines in Pakistan, should help improve the current system for registering medicines. Thus, this will enhance the availability of safe, effective and cost-effective generics for patients in Pakistan and other regions. This is starting to happen and will be monitored.
Competing interest: The authors declare that they have no conflicts of interest apart from those stated. No writing assistance was utilized in the production of this manuscript. The write-up of this paper was in part sponsored by the Karolinska Institutet.
Provenance and peer review: Not commissioned; externally peer reviewed.
Babar Khan1, BPharm, MPH, PhD
Brian Godman2,3, BSc, PhD
Ayesha Babar1, BPharm, MPhil
Shahzad Hussain4, BPharm, MPhil
Sidra Mahmood5, PharmD, MSc
Tahir Aqeel1, BPharm, MPhil
1Department of Pharmacy, University of Lahore, Jinnah Avenue, Islamabad Campus, Pakistan
2Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
3Strathclyde Institute of Pharmacy and Biomedical Sciences, Strathclyde University, Glasgow G4 ORE, UK
4National Institute of Health, House No. C13, NIH Colony, Chak Shahzad, Park Road, Islamabad, Pakistan
5Scotman Pharmaceuticals Pvt Ltd, 1-10/3, Industrial Area, Islamabad – 4400, Pakistan
References
1. Chowdhry NJ. Growth challenges of pharmaceutical industry in Pakistan. PPMA. Available from: http://health-asia.com/conferences/2014/Presentations/Day1/PharmaConvention/02-Nasir-javaid.pdf
2. European Commission (EC) Trade-related Technical Assistance Programme (TRTA) for Pakistan [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.tradecapacitypakistan.com/new/pdf/itc/SS2.pdf
3. Hafeez-Ur-Rehman. Manual of drug laws 2010. 2nd ed. Karachi: Pioneer Book House; 2010. 216-253.
4. Manual of Drug Laws. The Drug Act, 1976 [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://medisure.com.pk/drug_law/DrugAct+Rules/index.htm
5. Zaidi S, Bigdeli M, Aleem N, Rashidian A. Access to essential medicines in Pakistan: policy and health systems research concerns. PloS One. 2013;8(5):e63515.
6. World Health Organization. WHO good manufacturing practices for pharmaceutical products: main principles. Annex 2 [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.who.int/medicines/areas/quality_safety/quality_assurance/TRS986annex2.pdf
7. Jamshed SQ, Hassali MA, Ibrahim MI, Babar ZU. Knowledge attitude and perception of dispensing doctors regarding generic medicines in Karachi, Pakistan: a qualitative study. J Pak Med Assoc. 2011;61(1):80-3.
8. Jamshed SQ, Ibrahim MI, Hassali MA, Masood I, Low BY, Shafie AA, et al. Perception and attitude of general practitioners regarding generic medicines in Karachi, Pakistan: a questionnaire based study. South Med Rev. 2012;5(1):22-30.
9. Riaz H, Godman B, Hussain S, Malik F, Mahmood S, Shami A, Bashir S. Prescribing of bisphosphonates and antibiotics in Pakistan: challenges and opportunities for the future. J Pharm Health Serv Res. 2015;6:111-21.
10. Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166(3):332-7.
11. Corrao G, Soranna D, La Vecchia C, Catapano A, Agabiti-Rosei E, Gensini G, et al. Medication persistence and the use of generic and brand-name blood pressure-lowering agents. J Hypertens. 2014;32(5):1146-53.
12. Simoens S, Sinnaeve PR. Patient co-payment and adherence to statins: a review and case studies. Cardiovasc Drugs Ther. 2014;28(1):99-109.
13. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300(21):2514-26.
14. Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Internal Med. 2014;161(6):400-7.
15. Paton C. Generic clozapine: outcomes after switching formulations. Br J Psychiatry. 2006;189(2):184–5.
16. Veronin MA. Should we have concerns with generic versus brand antimicrobial drugs? A review of issues. J Pharm Health Serv Res. 2011;2(3):135-50.
17. Godman B, Wilcock M, Martin A, Bryson S, Baumgärtel C, Bochenek T, et al. Generic pregabalin; current situation and implications for health authorities, generics and biosimilars manufacturers in the future. Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(3):125-35. doi:10.5639/gabij.2015.0403.028
18. Corrao G, Soranna D, Arfe A, Casula M, Tragni E, Merlino L, et al. Are generic and brand-name statins clinically equivalent? Evidence from a real data-base. Eur J Intern Med. 2014;25(8):745-50.
19. Baumgärtel C, Godman B, Malmström R, Andersen M, et al. What lessons can be learned from the launch of generic clopidogrel? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68. doi:10.5639/gabij.2012.0102.016
20. Godman B, Baumgärtel C. Are generic immunosuppressants safe and effective? BMJ. 2015;350:h3248.
21. The Gazette of Pakistan. 13 November 2012 [homepage on the Internet]. [cited 2016 Nov 8]. Available from: www.na.gov.pk/uploads/documents/1352964021_588.pdf
22. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH guidelines [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.ich.org/products/guidelines.html
23. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). CDT. M4 : The common technical document [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.ich.org/products/ctd.html
24. Ramesh T, Saravanan D, Khullar P. Regulatory perspective for entering global pharma markets. Pharma Times. 2011;43(9):15-20.
25. Ikram J. Manufacturers say export of medicines declining. DAWN. 4 July 2015.
26. Drug Regulatory Authority of Pakistan. Updated list of pharmaceuticals manufacturers [homepage on the Internet]. [cited 2016 Nov 8]. Available from: www.dra.gov.pk/
27. Aamir M, Khalid Zaman. Review of Pakistan pharmaceutical industry: SWOT analysis. Int J Bus Inform Tech. 2011;1(1):114-7.
28. Analgesics in Pakistan. Euromonitor International. Sep 2016.
29. U.S. Pharmacopeial Convention. USP Ibuprofen updated list of pharmaceuticals manufacturers [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/errata467Ibuprofen.pdf
30. Zolner W. Critical aspects of HPLC. Eagle Analytical Services. November 2014.
31. 2012 Pakistan fake medicine crisis. Medicines. 25 July 2012.
32. Rias A. Pharmaceuticals: drug or poison. theHealth. 2012;3(2):36-8.
33. Implementation status of CTD, eCTD, and paper-free submissions: a global overview. Thomson Reuters.
34. The Jordanian Association of Pharmaceutical Manufacturers [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.japm.com/Public/English.aspx?Lang=2&Page_Id=194&Menu_ID=6&Menu_Parent_ID=-1&type=R
35. Jordan Economic and Commerce Bureau [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.jordanecb.org/Public/English.aspx?Site_Id=1&Page_Id=551&menu_id=38
36. Indian Council for Research on International Economic Relations. Ahmed V, Batool S. India-Pakistan trade: a case study of the pharmaceutical sector. Working paper 291 [homepage on the Internet]. [cited 2016 Nov 8]. Available from: http://www.slideshare.net/vahmed/indiapakistan-trade-a-case-study-of-the-pharmaceutical-sector
37. Mahmood KT, Amin F, Tahir M, Haq IU. A need for best patient care in Pakistan. A review. J Pharm Sci & Res. 2011;3(11);1566-84.
38. Stopping fake drugs from Pakistan is too late for victims. Bloomberg.
39. Riaz H, Godman B, Bashir S, Hussain S, Mahmood S, Malik F, et al. Evaluation of drug use indicators for non-communicable diseases in Pakistan. Acta Pol Pharm. 2016;73(3):787-94.
40. Shaikh BT, Ejaz I, Mazhar A, Hafeez A. Resource allocation in Pakistan’s health sector: a critical appraisal and a path toward the Millennium Development Goals. World Health Popul. 2013;14(3):22-31.
|
Author for correspondence: Brian Godman, BSc, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK; Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, SE-14186 Stockholm Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2016 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/assessment-of-active-pharmaceutical-ingredients-in-drug-registration-procedures-in-pakistan-implications-for-the-future.html
Author byline as per print journal: Brian Godman1,2,3, BSc, PhD; Eleonora Allocati4, BSc, MSc; Evelien Moorkens5, BSc, MSc
|
Abstract: |
Submitted: 9 September 2019; Revised: 12 September 2019; Accepted: 12 September 2019; Published online first: 25 September 2019
Brian Godman, Eleonora Allocati and Evelien Moorkens review the paper by Siu et al. regarding the evolving regulatory and reimbursement landscape in Canada [1], and subsequently link these within the broader global context of biosimilar market access to stimulate future activities and debates in this important area. This includes the current and potential market value of biosimilars, their regulatory and reimbursement environments as well as ongoing initiatives across countries to enhance their utilization to maximize potential savings. In addition, the potential sustainability of appreciable discounts among both originator and biosimilar manufacturers is discussed.
Siu et al. point out that biologicals are now commonly prescribed medicines for a wide variety of disease areas including diabetes, immunological diseases such as arthritis, inflammatory bowel disease and psoriasis, as well as for a number of cancers [1–4]. The high prices for new cancer medicines coupled with the growing prevalence of patients with cancer have resulted in growing (and now considerable) sales for oncology medicines across Europe and globally. Worldwide sales of cancer medicines, many of which are biologicals, was US$107 billion in 2015 and rising [5, 6]. The global anti-tumour necrosis factor (ant-TNF) market was valued at US$40.4 billion in 2017, and expected to expand at 2.54% per year with Europe and the US currently accounting for 87.70% of sales [7]. Within this, worldwide sales of adalimumab were US$17.6 billion in 2017, rising to nearly US$20 million in 2018 thereby making it the world’s best-selling medicine, with infliximab sales at US$5.9 billion in 2017 and etanercept at US$5.8 billion in 2017 [8, 9]. In Canada in 2017, biologicals accounted for 21.60% of overall public healthcare expenditure with the anti-TNF medicines accounting for 8.20% of total expenditure [1]. This compares with the US where biologicals currently account for 37% of net drug spending [10]. In the UK, anti-TNF medicines are also one of the highest spend areas within high-cost medicines, with GBP 780 million spent in England in 2018 [11], and in Germany, adalimumab was the top-selling medicine in 2017 with net costs to the statutory health insurance system of Euros 975 million [12]. Consequently, the introduction of biosimilars, especially in oncology and immunological diseases, should be of considerable interest to payers of health care and patients worldwide. We have seen limited use to date of biological medicines in many Central and Eastern European countries versus Western European countries due to their high costs and co-payments [13–15]. Consequently, biosimilars should help address this as prices fall. A competitive market should also bring down the cost of biosimilar insulins, helping many patients in low- and middle-income countries currently denied such treatments [16].
As a result of the current and envisaged sales of biological medicines coupled with ongoing initiatives, we are seeing considerable growth in the availability and use of biosimilars especially in China and Europe [17–20]. This will help increase their worldwide sales from approximately US$4 billion in 2018 [21]. Competition, with the resultant impact on overall expenditures, should intensify as many biological medicines that are currently used first- or second-line in treatment regimens lose their patent benefitting patients and payers [22].
Siu et al. point out appreciable changes in the regulatory and Health Technology Assessment (HTA) domains in the biosimilar environment in Canada in recent years to enhance their availability and promote their uptake [1]. This includes Health Canada in 2015 launching a pilot programme to provide manufacturers with the ability to discuss their biosimilar with Health Canada. In 2017, Health Canada laid out a Regulatory Review of Drugs and Devices which included a project to improve access to biologicals (biosimilars and non-biosimilars) by increasing their regulatory review capacity [1]. This should result in a more secure supply of biological drugs and more affordable biologicals. In addition, Health Canada and HTA organizations in Canada as well as the Institut national d’excellence en santé et en services sociaux (INESSS) in Quebec began collaborating in 2018 to better align review processes including biosimilars to reduce duplication and time lags between regulatory approval and reimbursement [1]. Heath Canada also intends to implement an updated naming convention for biologicals including the product’s brand name, International Nonproprietary Name (INN), and the Drug Identification Number (DIN), to support a clear distinction between biologicals including biosimilars to enhance adverse event tracking [1]. There have also been initiatives in other countries to enhance earlier access to biosimilars [20, 21, 23–26] although further developments are being proposed [27]. Interestingly, whilst modifications to the manufacturing process of originator biological drugs are common, regulators have very rarely required clinical studies to assess similarities even in the case of major manufacturing changes [28, 29].
With respect to reimbursing biosimilars, a number of significant changes have taken place recently in Canada to ease the situation. In Canada, the CADTH (Canadian Agency for Drugs and Technologies in Health) Common Drug Review (CDR) plays an important role in deciding whether medicines will be eligible for public reimbursement, with the provinces subsequently typically making the final decision based on CDR recommendations [30]. In May 2019, CADTH announced that as of 1 June 2019, it would no longer routinely review biosimilars via its CDR and pCODR (pan-Canadian Oncology Drug Review) programmes to streamline access [1]. A similar situation is seen in Quebec with INESSS. The pan-Canadian Pharmaceutical Alliance (pCPA) subsequently uses its combined purchasing power to improve access and increase the cost-effectiveness of medicines, similar to cross country collaborations in Europe [31–34]. In 2016, pCPA launched a more comprehensive biosimilar policy and in 2018 released a Biologics Policy Directive in which biological drugs for which biosimilars are already reimbursed as well as any new biosimilar will not be considered for reimbursement unless there are transparent price reductions to the lowest list price, providing an exemplar to others [1]. In addition, the potential for tiered pricing in certain therapeutic areas, which is likely to lead to increasing discounts as more biosimilars are launched. Manitoba was the first Canadian Province to instigate tiered arrangements in which biological-naïve patients must first be prescribed a biosimilar or an approved biological where no biosimilar exists [1]. Private insurers in Canada are also now offering preferential coverage for biosimilars leading to average savings of CA$8,500 per participating member per year [1]. Such initiatives should help boost the use of biosimilars in Canada along with educational, awareness and other initiatives, with biosimilar use currently lagging behind Europe [1, 19].
Biosimilars Canada has also recently developed a centralized patient support service platform to assist manufacturers and patients [1]. Policies regarding switching should also help enhance the use of biosimilars in Canada along with collecting real-world evidence to help ally current fears [1]. There have been considerable concerns with patients being switched between an originator biological medicine and a biosimilar across countries as well as indication extrapolation especially with respect to the risk of immunogenicity-related safety issues and diminished efficacy [17, 35]. However, an appreciable number of studies have now shown that such risks are unchanged when switching between an originator and a biosimilar [11, 21, 36–44]. As a result, patient and physician organizations in Canada are now supporting non-medical switching [1], similar to initiatives among European countries such as France [45]. The growing body of evidence for biosimilars is also leading to suggestions to modify the current lengthy approval process and costs to enhance earlier access and strengthen competition [46]. There are though still concerns with switching among physicians that need to be addressed with education as these can negatively impact on their future utilization [47–49]. Additional monitoring of patient outcomes in routine clinical practice should help further reduce possible concerns as well as potentially reduce the need for comparative clinical efficacy evidence as more originators lose their patents. This should reduce the investment needed for developing new biosimilars, and combined with developments in manufacturing [50], should help reduce future prices.
A number of other initiatives have also recently been undertaken in Canada to enhance the utilization of biosimilars. This includes the pan-Canadian Oncology Biosimilars Initiative to enhance successful adoption of biosimilars in oncology. British Columbia launched its Biosimilars Initiative in May 2019 promoting switching, with the savings used to lower premiums and co-pays where pertinent [1]. Other provinces are likely to follow.
We have also seen initiatives among other countries to enhance the use of biosimilars. Moorkens et al. and Vogler et al. have summarized these for Europe as well as provided future guidance to further enhance their uptake [51, 52]. More recently, Simoens et al. gave guidance on additional measures that could be introduced in Europe to fully realize the potential of biosimilars [53]. We are also seeing prescribing targets for biosimilars among European countries including national frameworks [54–57]. However, there are still limited initiatives in some countries including Japan where currently no position statement regarding biosimilars has been included in treatment guidelines for any of the cancer societies [58]. This may change though with increasing pressure on resources and with the Japanese government now reviewing supportive measures for biosimilars [58]. Smeeding et al. recently highlighted a number of issues that payers in the US should consider as part of any strategy to increase the use of biosimilars [59].
Ongoing initiatives across countries, including both supply- and demand-side measures, have increased potential savings from biosimilars. Siu et al. suggest that by the third year of entry, potential savings from biosimilars in Canada could range from 13%–43% for acute use products such as granulocyte colony-stimulating factors (G-CSF) and erythropoietin (EPO) and 8%–43% for chronic-use products, e.g. anti-TNFs [1]. This is helped by price reductions for biosimilars in Canada ranging between 17% and 50% from the originator.
A number of European countries have introduced price-link policies for biosimilars to lower their prices and enhance savings, with other countries instigating measures, such as tendering to lower prices [52, 60, 61]. For instance, tendering among hospitals in Norway resulted in a 72% discount compared to its list price [60], and tendering in the UK will result in GBP 300 million (approximately US$386 million) savings from currently GBP 400 million-per-year (approximately US$514 million) spent on adalimumab [62]. In Germany, the current high use of adalimumab and anticipated savings resulted in biosimilars accounting for 28% of total prescriptions of adalimumab within eight weeks of launch [12]. In the US, it is estimated that biosimilars will reduce direct spending on biological drugs by US$54 billion from 2017 to 2026 [10, 63]; with savings likely to be higher with greater discounts than 20% to 30% currently seen [10]. Substantial discounts for biosimilars across Europe, greater than initial considerations [61], coupled with demand-side measures, have already resulted in their appreciably increased use in recent years. In some cases and countries in Europe, in view of their lower prices, biosimilars now account for the total market for EPO and G-CSF. This is especially the case among a number of Central and Eastern European countries [19].
However, there are concerns that originators are starting to substantially lower their prices potentially affecting future biosimilar availability and the sustainability of the marketplace as seen recently with adalimumab [9, 19, 64]. In addition, we are seeing originator companies defending manufacturing and other patents, as well as seeking to instigate hurdles in the US making it more difficult for insurers to place biosimilars on formularies in order to disrupt the biosimilar market [10, 65, 66]. Originator companies are also developing new formulations of their biologicals to try and further disrupt the biosimilar market building on previous evergreening tactics [19, 67]. There are also suggestions to lower the prices of originators in countries such as Belgium and the US over time, negating the need for biosimilars to further interfere with this market [68]. We will continue to monitor these developments and their implications.
In conclusion, Siu et al. have provided a comprehensive review of current and planned policies in Canada to enhance the use of biosimilars at competitive prices to benefit payers and patients. This is important for disease areas such as cancer with ever increasing prices for new medicines, which potentially threaten the sustainability of healthcare systems [69, 70]. It is also increasingly likely that health authorities will start reassessing prices and potential discounts for on-patent medicines for oncology and immunological diseases as more standard medicines used for pricing negotiations lose their patents [6, 71]. Siu et al. also remind key stakeholders to continually monitor developments with biosimilars including both supply- and demand-side initiatives as well as encourage countries to learn from each other to enhance their uptake. This is critical for health authorities with the instigation of disruptive tactics such as in The Netherlands with AbbVie and in the US with hurdles such as rebates and other strategies with insurers to limit biosimilar use. In addition, payers need to monitor the development of new formulations by originator manufacturers and plan for the implications.
There was no funding for this Commentary.
Competing interests: The authors have no conflicts of interest to declare.
Provenance and peer review: Commissioned; internally peer reviewed.
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK
2Division of Public Health Pharmacy and Management, School of Pharmacy, Faculty of Health Sciences, Sefako Makgatho Health Sciences University, Pretoria, South Africa
3Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden
4Istituto di Ricerche Farmacologiche ‘Mario Negri’ IRCCS, Milan, Italy
5KU Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium
References
1. Siu ECK, Tomalin A, West K, Anderson S, Wyatt G. An ever-evolving landscape: an update on the rapidly changing regulation and reimbursement of biosimilars in Canada. Generics and Biosimilars Journal (GaBI Journal). 2019;8(3):107-18. doi:10.5639/gabij.2019.0803.014
2. Ferro A, Boyce M. Biological therapies: a long way on from Jenner. Br J Clin Pharmacol. 2013;76(2):161-3.
3. Barker J, Girolomoni G, Egeberg A, Goncalves J, Pieper B, Kang T. Anti-TNF biosimilars in psoriasis: from scientific evidence to real-world experience. J Dermatolog Treat. 2019:1-7.
4. Kuenzig ME, Benchimol EI, Lee L, Targownik LE, Singh H, Kaplan GG, et al. The impact of inflammatory bowel disease in Canada 2018: direct costs and health services utilization. J Can Assoc Gastroenterol. 2019;2(Suppl 1):S17-S33.
5. IMS Institute for Healthcare Informatics. IMSH Institute global oncology trend report 2015 and 2020 report. June 2016 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.scribd.com/document/323179495/IMSH-Institute-Global-Oncology-Trend-2015-2020-Report.
6. Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, et al. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
7. Transparency Market Research. Global TNF Inhibitors Market – Snapshot. 2018 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.transparencymarketresearch.com/tnf-inhibitors-market.html.
8. PRNewswire. Global tumor necrosis factor inhibitors drug market, dosage, price & clinical pipeline outlook 2024. 2018.
9. Hoen E’t. Humiragate: AbbVie’s desperate attempts to keep its monopoly. Medicines Law & Policy. 2019.
10. Stiff KM, Cline A, Feldman SR. Tracking the price of existing biologics when drugs enter the market. Expert Rev Pharmacoecon Outcomes Res. 2019;19(4):375-7.
11. Chan A, Kitchen J, Scott A, Pollock D, Marshall R, Herdman L. Implementing and delivering a successful biosimilar switch programme – the Berkshire West experience. Future Healthc J. 2019;6(2):143-5.
12. Grubert N. Biosimilar adalimumab achieves record launch impact in Germany—but government still plans reforms to boost biosimilar usage. Available from: https://www.linkedin.com/pulse/biosimilar-adalimumab-achieves-record-launch-impact-still-grubert/.
13. Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological therapies in immune-mediated inflammatory diseases: can biosimilars reduce access inequities? Front Pharmacol. 2019;10:279.
14. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198-206.
15. Kostić M, Djakovic L, Šujić R, Godman B, Jankovic SM. Inflammatory bowel diseases (Crohn’s disease and ulcerative colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85-93.
16. Gotham D, Barber MJ, Hill A. Production costs and potential prices for biosimilars of human insulin and insulin analogues. BMJ Global Health. 2018;3(5):e000850.
17. O’Callaghan J, Barry SP, Bermingham M, Morris JM, Griffin BT. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol. 2019;75(1):1-11.
18. Migliavacca Zucchetti B, Nicolò E, Curigliano G. Biosimilars for breast cancer. Expert Opin Biol Ther. 2019:1-7.
19. European Commission. IQVIA Report 2018: The impact of biosimilar competition in Europe [home-page on the Internet]. [cited 2019 Sep 12]. Avail-able from: https://ec.europa.eu/docsroom/docu-ments/31642
20. Derbyshire M. Regulation of copy biologicals in China. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(2):75-6. doi:10.5639/gabij.2018.0702.015
21. Shina S. Pharma News. Top developments in biosimilars during 2018. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):32-9. doi:10.5639/gabij.2019.0801.004
22. Derbyshire M, Shina S. Patent expiry dates for biologicals: 2017 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(1):29-34. doi:10.5639/gabij.2018.0701.007
23. US Food and Drug Administration. Biosimilar product regulatory review and approval [home-page on the Internet]. [cited 2019 Sep 12]. Available from: https://www.fda.gov/files/drugs/published/Biosimilar-Product-Regulatory-Review-and-Approval.pdf
24. US Food and Drug Administration. Biosimilar product information. 2019 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
25. Pettit C. Biosimilars market is ripe for cost savings. The Center for Biosimilars. 2019 Jun 27.
26. European Medicines Agency. Guideline on similar biological medicinal products. 23 October 2014 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf
27. Niazi SK. Rationalizing FDA guidance on biosimilars — expediting approvals and acceptance. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(2):84-91. doi:10.5639/gabij.2018.0702.018
28. Jiménez-Pichardo L, Gázquez-Pérez R, Sierra-Sánchez JF. Degree of prescriber’s knowledge about variability in biological drugs ‘‘innovators’’ in manufacturing process. Eur J Clin Pharmacol. 2018;74(4):505-11.
29. Vezér B, Buzás Z, Sebeszta M, Zrubka Z. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin. 2016;32(5):829-34.
30. CADTH Evidence Driven. CADTH Common Drug Review (CDR). 2019 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.cadth.ca/about-cadth/what-we-do/products-services/cdr
31. An Roinn Sáinte Department of Health. Ireland to open negotiations with Belgium, the Netherlands, Luxembourg and Austria on drug pricing and supply – Minister Harris. 2018.
32. European Observatory. How can voluntary cross-border collaboration in public procurement improve access to health technologies in Europe? [homepage on the Internet]. [cited 2019 Sep 12]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0009/331992/PB21.pdf?ua=1
33. Henrard S, Arickx F. Negotiating prices of drugs for rare diseases. Bull World Health Organ. 2016;94(10):779-81.
34. O’Mahony JF. Beneluxa: what are the prospects for collective bargaining on pharmaceutical prices given diverse health technology assessment processes? Pharmacoeconomics. 2019;37(5):627-30.
35. Aladul MI, Fitzpatrick RW, Chapman SR. Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: a qualitative study. BMJ Open. 2018;8(11):e023603.
36. Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463-78.
37. Gisondi P, Bianchi L, Calzavara-Pinton P, Conti A, Chiricozzi A, Fimiani M, et al. Etanercept biosimilar SB4 in the treatment of chronic plaque psoriasis: data from the Psobiosimilars registry. Br J Dermat. 2019;180(2):409-10.
38. Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304-16.
39. Stebbing J, Baranau YV, Baryash V, Manikhas A, Moiseyenko V, Dzagnidze G, et al. Double-blind, randomized phase III study to compare the efficacy and safety of CT-P6, trastuzumab biosimilar candidate versus trastuzumab as neoadjuvant treatment in HER2 positive early breast cancer (EBC). J Clin Oncol. 2017;35(15_suppl):510-.
40. Esteva FJ, Stebbing J, Wood-Horrall RN, Winkle PJ, Lee SY, Lee SJ. A randomised trial comparing the pharmacokinetics and safety of the biosimilar CT-P6 with reference trastuzumab. Cancer Chemother Pharmacol. 2018;81(3):505-14.
41. Komaki Y, Yamada A, Komaki F, Micic D, Ido A, Sakuraba A. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-alpha agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45(8):1043-57.
42. Pegram MD, Bondarenko I, Zorzetto MMC, Hingmire S, Iwase H, Krivorotko PV, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer. 2019;120(2):172-82.
43. Waller CF, Ranganna GM, Pennella EJ, Blakeley C, Bronchud MH, Mattano LA, Jr., et al. Randomized phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H in the prophylactic treatment of chemotherapy-induced neutropenia. Ann Hematol. 2019;98(5):1217-24.
44. Tweehuysen L, Huiskes VJB, van den Bemt BJF, Vriezekolk JE, Teerenstra S, van den Hoogen FHJ, et al. Open-label, non-mandatory transitioning from originator etanercept to biosimilar sb4: six-month results from a controlled cohort study. Arthritis Rheumatol. 2018;70(9):1408-18.
45. GaBI Online – Generics and Biosimilars Initiative. Policies & Legislation – France aims to reach 80% biosimilar penetration by 2022 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Sep 12]. Available from: http://gabionline.net/Policies-Legislation/France-aims-to-reach-80-biosimilar-penetration-by-2022
46. Frapaise FX. The end of phase 3 clinical trials in biosimilars development? BioDrugs. 2018;32(4):319-24.
47. Teeple A, Ellis LA, Huff L, Reynolds C, Ginsburg S, Howard L, et al. Physician attitudes about non-medical switching to biosimilars: results from an online physician survey in the United States. Curr Med Res Opin. 2019;35(4):611-7.
48. Pineles D, Malter L, Liang PS, Arsuaga A, Bosworth B, Hudesman DP, et al. The nocebo effect and patient perceptions of biosimilars in inflammatory bowel disease. Eur J Clin Pharmacol. 2018;74(10):1361-2.
49. Smolen JS, Goncalves J, Quinn M, Benedetti F, Lee JY. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5(1):e000900.
50. DiMasi JA, Smith Z, Getz KA. Assessing the financial benefits of faster development times: the case of single-source versus multi-vendor outsourced biopharmaceutical manufacturing. Clin Ther. 2018;40(6):963-72.
51. Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PloS One. 2017;12(12):e0190147.
52. Vogler S, Schneider P. Do pricing and usage-enhancing policies differ between biosimilars and generics? Findings from an international survey. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(2):79-88. doi:10.5639/gabij.2017.0602.015
53. Simoens S, Le Pen C, Boone N, Breedveld N, Celano A, Llombart-Cussac A, et al. How to realize the potential of off-patent biologicals and biosimilars in Europe? Guidance to policymakers. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(2):70-4. doi:10.5639/gabij.2018.0702.014
54. Health Improvement Scotland. Biosimilar medicines: a national prescribing framework. 2018 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: http://www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/programme_resources/biosimilar_medicines_framework.aspx
55. All Wales Medicine Strategy Group. National Prescribing Indicators 2018–2019. 2018 [home-page on the Internet]. [cited 2019 Sep 12]. Available from: http://www.awmsg.org/docs/awmsg/medman/National%20Prescribing%20Indicators%202018-2019.pdf
56. NHS Scotland. Secondary Care National Therapeutic Indicators 2018/19 [homepage on the Internet]. [cited 2019 Sep 12]. Available from: https://www.therapeutics.scot.nhs.uk/wp-content/uploads/2018/08/Secondary-Care-National-Therapeutic-Indicators-Version-1.0.pdf
57. Underwood G. The biosimilars era. Pharma Times. 2018.
58. Hara F, Tajima K, Tanabe K. Current situation and challenges regarding biosimilars in Japan: an example of trastuzumab biosimilars for breast cancer. Future Oncol. 2019;15(12):1353-61.
59. Smeeding J, Malone DC, Ramchandani M, Stolshek B, Green L, Schneider P. Biosimilars: considerations for payers. PT. 2019;44(2):54-63.
60. Matusewicz W, Godman B, Pedersen HB, Fürst J, Gulbinović J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):755-8.
61. Godman B. Health authority perspective on biosimilars. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):10-11. doi:10.5639/gabij.2013.0201.010
62. Davio K. After Biosimilar deals, UK spending on adalimumab will drop by 75%. 2018. The Center for Biosimilars.
63. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3.
64. Sagonowsky E. AbbVie’s massive Humira discounts are stifling Netherlands biosimilars: report. 2019 Apr 2. FiercePharma.
65. Reinke T. Show us (the U.S.) the savings. Manag Care. 2019;28(1):9-10.
66. Kellaher C. AbbVie, Boehringer settle U.S. patent dispute over drug Humira. The Wall Street Journal. 2019 May 14.
67. Vernaz N, Haller G, Girardin F, Huttner B, Combescure C, Dayer P, et al. Patented drug extension strategies on healthcare spending: a cost-evaluation analysis. PLoS Med. 2013;10(6):e1001460.
68. Atteberry P, Bach PB, Ohn JA, Trusheim M. Biologics are natural monopolies (Part 1): why biosimilars do not create effective competition. Health Affairs Blog. 2019 [cited 2019 Sep 12]. Available from: https://www.healthaffairs.org/do/10.1377/hblog20190405.396631/full
69. Godman B, Wild C, Haycox A. Patent expiry and costs for anti-cancer medicines for clinical use. Generics and Biosimilars Initiative Journal. 2017;6(3):105-6. doi:10.5639/gabij.2017.0603.021
70. Ghinea H, Kerridge I, Lipworth W. If we don’t talk about value, cancer drugs will become terminal for health systems. The Conversation. 2015 Jul 28.
71. Godman B, Hill A, Simoens S, Kurdi A, Gulbinovič J, Martin AP. Pricing of oral generic cancer medicines in 25 European; findings and implications. Generics and Biosimilars Initiative Journal. 2019;8(2):49-70. doi:10.5639/gabij.2019.0802.007
|
Author for correspondence: Brian Godman, BSc, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2019 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/ever-changing-landscape-of-biosimilars-in-canada-findings-and-implications-from-a-global-perspective.html
Author byline as per print journal: Christoph Baumgärtel, MD, MSc; Brian Godman, BSc, PhD
|
Abstract: |
Submitted: 29 September 2015; Revised: 19 October 2015; Accepted: 19 October 2015; Published online first: 2 November 2015
Regulatory bioequivalence rules for usual generics are well established and already recognized. However, for narrow therapeutic index drugs and immunosuppressives, there are specific and tighter criteria in place.
Drugs with a narrow therapeutic index are defined by a narrow distance between the dosage that induces a desired effect and that dosage which already has a toxic effect. Typically, this ratio in the field of pharmacology is indicated by the quotient of LD50/ED50 (LD50 = the dose at which 50% of the animals die, ED50 = the dose at which 50% of the animals show the desired effect). Alternatively, the quotient of LD5/ED95 can be used, which can better illustrate a non-linear dose-response curve.
For a perfectly safe drug, the ratio should therefore be very high. If in contrast the ratio is low, i.e. if a drug shows a value of only 3 or 4, this is called a ‘narrow therapeutic index drug’ (NTI), which must always be dosed with particularly high accuracy. Even a minor variation in plasma levels may lead sometimes to treatment failure on the one hand or inevitably to toxic effects on the other. Examples of such agents typically include immunosuppressants, digitalis, theophylline and some anti-epileptic drugs.
The acceptance range of bioequivalence trials which is usually applied for a marketing authorization of a generic drug is 80–125% of the 90% confidence interval of the ratio of the test and reference products’ AUC (area under the curve) and Cmax (maximum plasma concentration). For drugs with a narrow therapeutic index, and especially for immunosuppressives, the European Medicines Agency (EMA) demands even greater accuracy in justified cases and therefore has set more stringent criteria. This is despite in practice ratios for authorized conventional generics usually differ on average by only three to four per cent from their originator [1–4].
EMA’s overhauled bioequivalence guideline [5], in force since 2010, requires that for potential narrow therapeutic index drugs the EMA’s Pharmacokinetic Working Party (PKWP) [6] will evaluate if a generic drug newly submitted for authorization is to be thought of as an NTI and whether for this NTI actually stricter bioequivalence criteria have to be applied. It is important for a pharmaceutical manufacturer or applicant to know that there is no precasted list that names all such agents, but that all agents submitted for generic drug authorization will be evaluated by the authority on a case-by-case basis with regard to their NTI requirements.
Examples where this has already been practised are the regulatory requirements for ciclosporin and tacrolimus generics as described in a PKWP Questions and Answers document published on the EMA website [7]. In the case of these two immunosupressives, restricted bioequivalence criteria were set.
For ciclosporin, narrower acceptance intervals of 90.00–111.11% are required for both the AUC and the Cmax. Whereas, for tacrolimus the narrow acceptance interval is only required for the AUC but is not required for the Cmax. This is because tacrolimus plasma levels show accumulation with repeated dosing, resulting in a lower relevance being given to differences in initial peak plasma concentrations..
The narrower acceptance range limits for the confidence intervals for NTIs, see Figure 1, provide a greater confidence in the true bioequivalence for these drug substances. However, this requirement significantly increases the number of subjects necessary for the bioequivalence studies. Tacrolimus, for example, is a drug which is not merely an NTI, but additionally shows relatively high intra-individual variation in plasma levels. It has a relatively high coefficient of variation, close to 30%, which would classify it as a highly variable drug. Even in bioequivalence trials of tacrolimus, when a conventional acceptance range is applied, this would typically necessitate enrolling substantially higher numbers of trial participants than in the usual, bioequivalence guideline requiring a minimum of 12 to 24 subjects. One would need at least 40 subjects for a tacrolimus product, and in fact to demonstrate compliance with EMA’s mandatorily required narrower acceptance limits, it might require up to 200 to 300 subjects.
To increase the safety of generic immunosuppressives even more, it is also recommended that the summary of product characteristics (SPC) states that patients who are prescribed either a generic immunosuppressant after an originator or are switched in any other way, have their plasma levels monitored during the time of the switch to avoid potential rejection [8]. This is however similar to what is undertaken in normal clinical practice when patients are first placed on an immunosuppressant after receiving a solid organ graft.
Because of the issues concerning generic immunosuppressive medicines, Molnar et al. recently undertook a systematic review and meta-analysis of all available studies since 1980 comparing generic with originator (innovator) immunosuppressive medicines [9]. The authors documented that acute rejection was rare in transplant patients given generic immunosuppressive medicines and the incidence of rejection did not differ between the groups. However, as recently stated, the methodological standard of the published studies included was very variable and follow-up times were short [10].
In the evaluation of the pooled pharmacokinetic data, Molnar et al. showed that the generics met the US Food and Drug Administration (FDA) bioequivalence criteria, but did not all meet the stricter EMA criteria [9]. It appears that the small number of patients in some of the included studies, and as a result the wide confidence intervals, significantly contributed to this finding. For statistical reasons, in order for results to meet the stricter acceptance criteria for immunosuppressives, requiring narrow confidence intervals, a sufficiently higher number of patients must be included in the trials [11].
This effect on subject numbers needed is illustrated by the wide confidence intervals found in immunosuppressive studies with less than 20 subjects. As reviewed in the paper by Molnar et al., only trials with approximately 50 to 70 patients were able to fulfil the EMA acceptance criteria [9]. In detail, their sub-analysis of two randomized kidney trials showed that with a mean of 30 subjects, both failed to fulfil the stricter EMA bioequivalence criteria, whereas the pooled sub-analysis of seven non-randomized interventional kidney studies with a 53% higher mean sample size of 46 patients did fulfil these criteria.
Notably, the mean ratios of the test and reference products’ AUC and Cmax in most of the reported trials were well within the expected range [12]; and were in fact only a few percentage points higher or lower than 100% [9]. These data strongly suggest that there are no clinically important problems with generic immunosuppressive agents, especially with those that meet the EMA criteria, but rather that there are problems with the scientific value, relevance and interpretation of smaller studies.
It should also be noted that EMA’s precautious narrowing of bioequivalence limits was specifically implemented for situations where it is suspected that plasma level monitoring will – against the SPC advice – not be complied with; such as following a switch from an originator to a generic drug [7]. This narrowing can therefore be seen as a ‘safety net’ for the use of immunosuppressive generics. This suggests that generic immunosuppressive drugs, used in the correct manner by practitioners aware of their precautions, especially the requirement for monitoring of plasma levels at the time of switching, may indeed be considered to be bioequivalent and expected to produce outcomes that are similar to those produced by originator products.
This expectation is supported by the fact that generic versions of immunosuppressive medicines, i.e. ciclosporin, have been on the market in Europe for more than 10 years and authorities’ pharmacovigilance systems have not identified any serious issues specific for generic immunosuppressives, even after an estimated hundreds of thousands of prescribed and dispensed doses. This should alleviate major concerns among clinicians and patients when considering or undertaking a switch. However, it is expected that further well-designed studies with a suitable number of patients will help to fully address any remaining concerns with generic immunosuppressives. Further education among physicians about the need to reliably moni tor blood levels in patients when first prescribed generic immunosuppressives will also be needed. Such activities may also help to enhance adherence to immunosuppressive medicines, which is a crucial concern in transplant patients [13].
Competing interest: None.
Provenance and peer review: Not Commissioned; externally peer reviewed.
Christoph Baumgärtel, MD, MSc, Senior Scientific Expert, Coordination Point to Head of Agency, AGES Austrian Medicines and Medical Devices Agency and Austrian Federal Office for Safety in Health Care, EMA European Expert, Vice Chair of Austrian Prescription Commission, 5 Traisengasse, AT-1200 Vienna, Austria
References
1. American Medical Association. Featured report: generic drugs (A-02), June 2002 AMA Annual Meeting [homepage on the Internet]. [cited 2015 Oct 19]. Available from: http://www.ama-assn.org/ama/pub/about-ama/our-people/ama-councils/council-science-public-health/reports.page?
2. Henney JE. From the Food and Drug Administration. JAMA. 1999;282(21):1995
3. Nwakama PE. Generic drug products demonstrate small differences in bioavailability relative to brand name counterparts: Review of approved ANDAs, FDA. 2015
4. Davit BM, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583-97.
5. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on the investigation on bioequivalence. EMA: CPMP/EWP/QWP/1401/98 Rev. 1. January 2010 [homepage on the Internet]. 2010 Mar 10 [cited 2015 Oct 19]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf
6. European Medicines Agency. Pharmacokinetics Working Party [homepage on the Internet]. [cited 2015 Oct 19]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/contacts/CHMP/people_listing_000070.jsp&mid=WC0b01ac05802327c9
7. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Questions & answers: postitions on specific questions addressed to the Pharmacokinetics Working Party (PKWP). EMA/618604/2008 Rev. 12. 25 June 2015 [homepage on the Internet]. 2015 Jul 20 [cited 2015 Oct 19]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002963.pdf
8. Austrian Federal Office for Safety in Health Care. Austrian Medicines and Medical Devices Agency. Available from [homepage on the Internet]. [cited 2015 Oct 19]. Available from: https://aspregister.basg.gv.at/aspregister/
9. Molnar AO, et al. Generic immunosuppression and solid organ transplantation: systematic review and meta-analysis. BMJ. 2015;350:h3163.
10. Godman B, Baumgärtel C. Are generic immunosuppressants safe and effective? BMJ. 2015;350:h3248.
11. Baumgärtel C. [Bioequivalence – narrow therapeutic index drugs]. Bioäquivalenz – Arzneimittel mit enger therapeutischer Breite. ÖAZ, Österreichische Apotheker Zeitung. 2012;66(23):60-1. German.
12. Baumgärtel C. Myths, questions, facts about generic drugs in the EU. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):34-8. doi:10.5639/gabij.2012.0101.009
13. Tong A, Howell M, Wong G, Webster AC, Howard K, Craig JC. The perspectives of kidney transplant recipients on medicine taking: a systematic review of qualitative studies. Nephrol Dial Transplant. 2011;26(1):344-54.
|
Author for correspondence: Brian Godman, BSc, PhD, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/bioequivalence-of-narrow-therapeutic-index-drugs-and-immunosuppressives.html
Author byline as per print journal: Brian Godman, BSc, PhD; Michael Wilcock, MPharm; Andrew Martin, MPharm; Scott Bryson, MSc, MPH; Christoph Baumgärtel, MD; Tomasz Bochenek, MD, MPH, PhD; Winne de Bruyn, BSc; Ljiljana Sović Brkičić, MPharm; Marco D’Agata, MSc; Antra Fogele, PhD; Anna Coma Fusté, MSc; Jessica Fraeyman, PhD; Jurij Fürst, MD; Kristina Garuoliene, MD, PhD; Harald Herholz, MD, MPH; Mikael Hoff mann, MD, PhD; Sisira Jayathissa, MBBS, MMedSc (Clin Epi), MD, FRCP (Lond, Edin), FRACP, FAFPHM, FNZCPHM, DClinEpi, DOPH, DHSM, MBS; Hye-Young Kwon, BPharm, MPH, PhD; Irene Langner, MA; Marija Kalaba, MD; Eva Andersén Karlsson, MD, PhD; Ott Laius, PhD; Vanda Markovic-Pekovic, PhD; Einar Magnusson, MD; Stuart McTaggart, MSc; Scott Metcalfe, MBChB, DComH, FAFPHM (RACP), FNZCPHM; Hanne Bak Pedersen, MD; Jutta Piessnegger, PhD; Anne Marthe Ringerud, MPharm; Gisbert W Selke, BSc; Catherine Sermet, MD; Krijn Schiffers, BSc; Peter Skiold, MSc; Juraj Slabý, MD; Dominik Tomek, Pharm Dr, PhD, MPH; Anita Viksna, PhD; Agnes Vitry, PhD; Corinne Zara, MSc; Rickard E Malmström, MD, PhD
|
Introduction: The manufacturer of pregabalin has a second use patent covering prescribing for neuropathic pain – its principal indication. The manufacturer has threatened legal action in the UK if generic pregabalin rather than Lyrica is prescribed for this indication. No problems exist for practitioners who prescribe pregabalin for epilepsy or generalized anxiety disorder. This has serious implications for health authorities. In Germany, however, historically generics can be legally prescribed for any approved indication once one indication loses its patent. |
Submitted: 27 March 2015; Revised: 11 June 2015; Accepted: 24 August 2015; Published online first: 7 September 2015
The increased use of generic medicines is essential to sustain healthcare systems given the ever-increasing pressure on resources [1–4]. Prices of generic drugs are as low as 2–10% of pre-patent loss prices in some countries [5–7]. Consequently, increased use of generic drugs can generate substantial savings, which can be redirected into funding new valued high-priced medicines [2, 5–12], which is especially important for countries struggling to fund these medicines. A number of strategies globally have been initiated to encourage prescribing and dispensing of generic drugs rather than the originator (brand-name) drug, as well as patented products in a class in which all medicines are seen as essentially similar at therapeutically equivalent doses [4, 8–12].
Increasing use of generic drugs does not appear to compromise care, and many studies have reported little or no difference in outcomes across a range of products and classes [13–18]. In Europe, only generic drugs produced in accordance with the European Medicines Agency’s strict guidelines and definitions [19] are granted marketing authorization.
Well-known and agreed exceptions to generics prescribing or substitution include lithium, theophyllines, some anti-epileptic drugs, modified release preparations and immunosuppressants. In these cases, brand-name prescribing is endorsed [20–23]. Agreed exceptions to generics prescribing, including medicines to treat epilepsy and prevent organ rejection, also exist in Germany and Sweden [6, 24].
A new emerging problem, however, has come to the fore in recent years, concerning the expiry of patents for medicines that have patents for more than one indication, and the threat of legal action by the manufacturer of the originator drug against physicians. This situation occurred recently in the case of pregabalin for the treatment of epilepsy and generalized anxiety disorder (GAD) when the basic patent for pregabalin expired in July 2014 in a number of European countries. The patent for its second medical use, protecting the originator drug Lyrica’s use in treating neuropathic pain, extends to July 2017 in Europe [25, 26]. In the UK, this resulted in the manufacturer of the originator drug (Lyrica) claiming patent infringement and warning doctors not to prescribe the generic drug pregabalin for neuropathic pain [26, 27]. As far as we are aware, this is the first time this has happened, and has serious implications for health authorities.
Prior to this, the originator manufacturer of Lyrica had been fined heavily for promoting gabapentin (prelude to pregabalin) off label for the treatment of neuropathic pain [28–31], although it is now recommended for this indication [32]. In addition, there have been concerns with the methodological limitation of some of the studies of pregabalin in neuropathic pain [33–35]. Pregabalin, for example, is currently not listed in the ‘Wise List’ of Stockholm Metropolitan Healthcare Region because of efficacy and safety concerns compared with other treatments for these conditions [36]. However, there are increasing concerns with the implications of the activities of second use patents with Lyrica [26, 37].
In this paper, historical developments in Germany and the UK relating to this case are examined. Personnel from regional and national health authorities from principally across Europe, and advisers to health authorities working in universities, were then surveyed to ascertain the current situation with pregabalin in their country and to determine the best strategy for maximizing savings for countries once a product loses its patent for any indication.
In the UK, the international non-proprietary name (INN) prescribing rate is over 80%, and up to 98–99% of non-contentious generic drugs, such as proton pump inhibitors, renin-angiotensin inhibitors and statins, with pharmacists not permitted to substitute an originator drug with a generic drug when the originator drug is prescribed [7, 20, 21, 38].
The UK medicines agency recently issued advice on which epilepsy drugs to prescribe by brand name (originator) and which by INN [39]. Pregabalin was considered suitable for INN prescribing [39], which was endorsed by the originator company stating ‘there will be no clinical superiority of the originator branded medicine Lyrica over generic pregabalin’ [25].
The extended patent for neuropathic pain resulted in the originator company writing to all Clinical Commissioning Groups (CCGs) in England and Health Boards in Scotland in November 2014 pointing out that generics of pregabalin were expected to be approved only for GAD and epilepsy indications, and that the prescribing of generic pregabalin for neuropathic pain could represent ‘off-label’ use. This would be considered a patent infringement constituting an unlawful act, with the originator company reserving all legal rights in this regard [25–27].
The wish of generics companies to make generic pregabalin available in the UK across all indications resulted in a court case, with the originator company as claimant and the Actavis group as the principal defendant [26, 40]. The judge in his deliberations, posted on 21 January 2015, granted Actavis the possibility to launch generic pregabalin and again stated that the best way forward was to try to ensure physicians prescribe Lyrica for the treatment of neuropathic pain and pregabalin for other conditions, including epilepsy [40, 41].
The actions of the originator company are unsurprising. In 2013, global sales of Lyrica generated US$4.6 billion for the company [40]. In the UK, sales of Lyrica increased by 53% between 2011 and 2013 to about US$310 million. It is estimated that 54% of prescriptions in September 2014 were for treating pain, of which 44% was for neuropathic pain [40]. In 2014, sales of Lyrica were GBP 250 million (US$390 million) [26].The potential loss in revenue, therefore, would hugely impact company sales – estimated to be GBP 220 million per year (US$340 million) across all indications assuming high INN prescribing rates and generic drug prices rapidly falling by 90% of the price of Lyrica [7, 42].
In an attempt to preserve sales of Lyrica, the originator company has been proactive in lobbying groups in the UK who could influence physician prescribing, such as the Medicine Management groups within CCGs, the Pharmaceutical Services Negotiating Committee, the General Practitioners Committee of the British Medical Association, and the National Health Service [26, 32, 37, 43–45]. For instance, National Health Service (NHS) England in March 2015 issued advice to all CCGs that within electronic prescription systems there should be a notice or advice box stating ‘If treating neuropathic pain, prescribe Lyrica (brand) due to patent protection. For all other indications, prescribe generically’ [45]. The Pharmaceutical Services Negotiating Committee stated to its members they should be aware that the originator company still retains the indication for neuropathic pain. Members were also made aware that following a high court decision, ‘it was agreed by all parties that the generic [drug] producers would write to CCGs to ensure they were aware that the generic [drug] could not be supplied for the patented indication. A CCG or other party that promotes the supply of generic pregabalin for the patented indication risks facing legal action’ [43].
This situation in the UK has important future implications for generics and biosimilars companies across countries, as it may impede the ability of health authorities to fully realize potential savings from generics and biosimilars once the first indication loses its patent, especially if pharmaceutical companies look to extend the number of indications for their new medicines once launched in an attempt to extend the patent life.
Germany has taken a different approach to the UK. Currently, nine pregabalin generics are available and reimbursed in Germany (up to April 2015), all of which have the indications for epilepsy and anxiety disorders. The situation, however, is now less clear cut as the originator manufacturer, has taken Ratiopharm, Hexal, 1A Pharma, Glenmark and Aliud Pharma and some Sickness Funds (German payers) to court in an attempt to conserve Lyrica sales for neuropathic pain (up to 10 April 2015) [46]. The legal battle is still ongoing. The originator company’s previous strategy to promote Lyrica was to communicate directly with physicians or via KVs (regional doctors’ associations) by letter, making it clear that Lyrica was the only pregabalin licensed for neuropathic pain [47]. However, these communications were largely dismissed by KVs because the focus was on legal rather than medical issues, and the KVs continued to advise physicians to reach targets of generics prescribing of at least 85%. In addition, the Social Code Book V (SGB V), which is decisive for Sickness Funds, stated in paragraph 129 that generics substitution is possible wherever at least one indication matches [48–50].
The contrast between the situation in the UK and the situation in Germany, and the implications for potential savings when other pharmaceutical products lose their patents for some but not all indications, has led health authorities, across Europe, to review the current status of pregabalin in other countries in order to refine their own strategies if possible.
A qualitative study was undertaken to ascertain the current situation between generic pregabalin and Lyrica among health authorities principally from across Europe. This included a range of Central, Eastern and Western European countries with different epidemiology and funding of health care, as well as policies to enhance the prescribing of generics. This builds on the situation Germany and the UK, and is in line with current recommendations for conducting cross-national research projects [51]. The aim was to maximize future savings for countries once a product loses its patent for any indication.
Personnel from 33 regional and national health authorities mainly from across Europe, and personnel from nine universities working closely as advisers to health authorities or with insight into health authority activities, were contacted by email to provide answers to the following four questions (up to April 2015):
1. Are you aware of any similar examples to the situation of pregabalin and Lyrica in the UK from other pharmaceutical companies for small molecules once the patent has been lost (biosimilars are a different issue)? If so, what were these and how were they handled (if at all).
2. Was Lyrica reimbursed in your country? If yes, for what indications?
3. Has generic pregabalin been launched in your country/about to be launched? If yes what date (month) and indications?
4. Has the originator company issued a letter to healthcare professionals in your country similar to the letter issued to CCGs in the UK? If yes, what actions (if any) are being taken?
This was supplemented with knowledge from other high-income countries taking different approaches to the availability of generic pregabalin to potentially provide additional examples.
All health authority personnel are involved with either pricing and reimbursement decisions, decisions concerning funding or monitoring the use of medicines, or both, including generics, in their countries and regions. Consequently, it was felt that they would have the most insight into the current situation concerning pregabalin and Lyrica in their countries and regions. European countries included those from Central, Eastern and Western Europe to ensure legitimacy with the findings. Personnel from regions in The Netherlands, Sweden and the UK were also included, as healthcare budgets in these countries are devolved downwards.
The written information supplied by the co-authors and others for each of the questions for each country was collated and summarized by the corresponding author. The summarized information was subsequently checked via email and face-to-face contact with the relevant co-author(s) to ensure the accuracy of the summarized information. The information supplied was subsequently summarized into five categories to improve the interpretation of the findings and the implications for the future, building on the situation in England and Germany.
The five categories included:


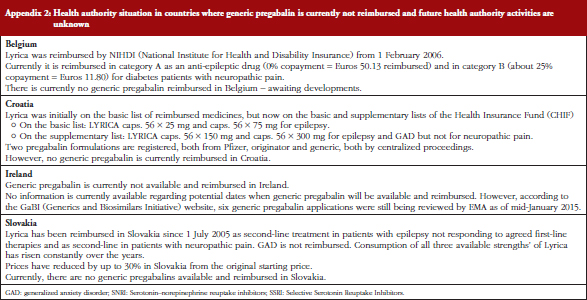
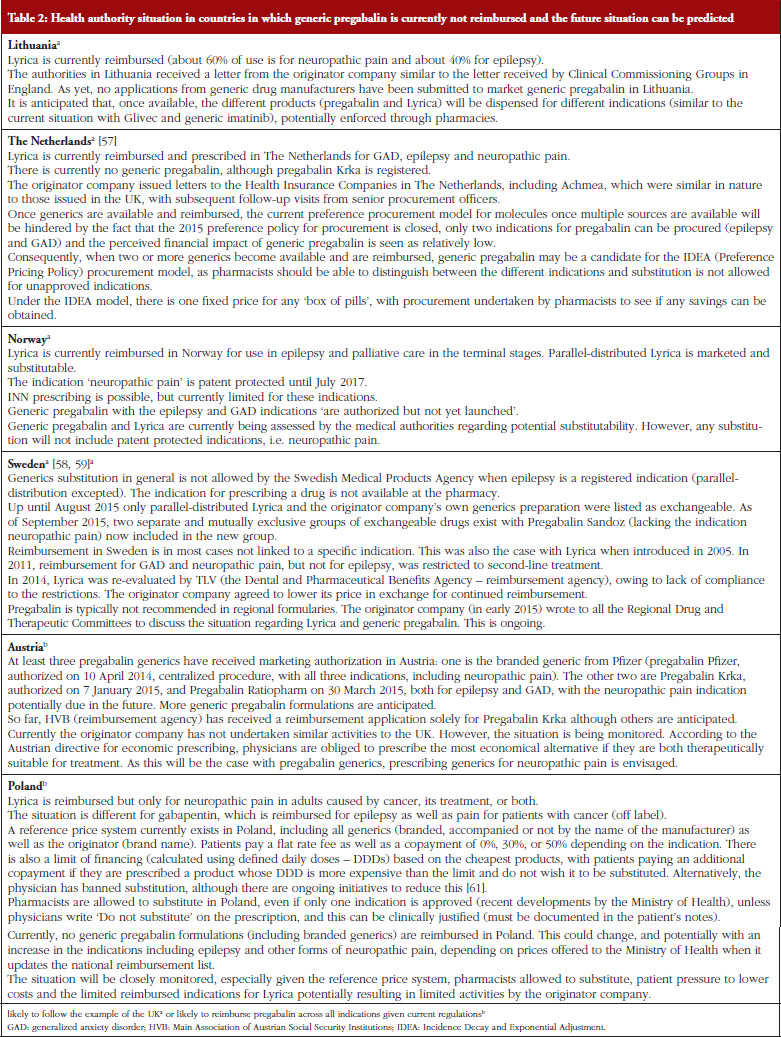
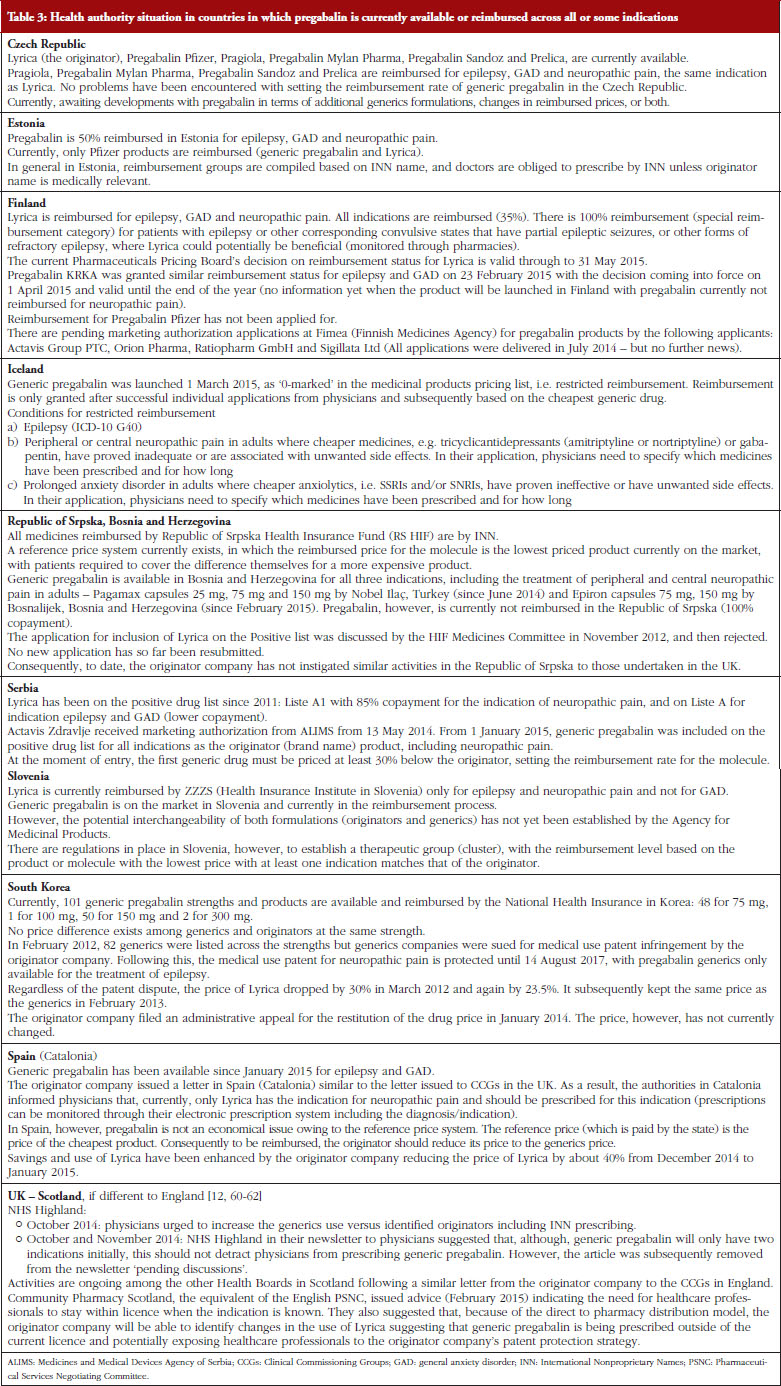
Potential or actual demand-side measures among the health authorities were not broken down into the ‘four Es’: education, engineering, economics and enforcement, as in our previous paper on generic clopidogrel [52]. This is because pregabalin may not be available and reimbursed across Europe and the other chosen countries.
This information was supplemented with a limited literature search for further information about generics generally, pregabalin and the activities of the originator company, including recent court cases, as well as relevant papers known to the co-authors. A similar methodological approach was used when reviewing health authority activities when generic clopidogrel became available [52].
The results of the survey revealed that respondents were typically unaware of similar examples to pregabalin and Lyrica in their countries. For example, generic clopidogrel was reimbursed and endorsed by health authority personnel from across Europe despite generic clopidogrel not including all licensed indications at launch [52]. The main exception was Lithuania, see Table 2, with Glivec and generic imatinib.
The current situation for Lyrica and generic pregabalin among health authorities and health insurance companies across Europe and other selected countries is included in Tables 1–3 as well as Appendices 1 and 2. This also includes additional activities in Scotland.
In this paper, we have described the situation across Europe following the launch or imminent launch and reimbursement of generic pregabalin. We were not surprised by the activities of the originator company in the UK in view of the current high levels of INN prescribing, no clinical issues with patients being switched between generic pregabalin or Lyrica across indications, and the high sales of Lyrica globally and in the UK [7, 21, 25, 39, 40, 65].
The threat of legal action against physicians taught to prescribe generically is a major concern among health authorities already struggling to fund increased volumes and new high-priced medicines within available budgets [66]. It also raises issues about off-label prescribing generally and pharmacists checking the use of medication with every patient [37]. Moreover, it would seem that this is the first time that an originator company has threatened court cases against physicians in an extended patent use situation. Previous examples can be found in some countries such as Lithuania, see Table 2; however, no coordinated approach has been taken across countries. These concerns are exacerbated if such activities make European markets unattractive for generics companies, thereby reducing potential savings once a product loses its patent. It is also unhelpful to influence physicians to remember to prescribe different versions of the same molecule for different indications. This could, however, potentially be addressed through increasing use of electronic prescribing support systems. Actions of this nature also impede constructive working relationships between pharmaceutical companies and health service personnel [26].
As seen in Tables 1–3, and Appendices 1 and 2, very different approaches have been taken across countries to the availability of generic pregabalin. In addition to historic approaches taken in Germany, countries such as Czech Republic, Estonia, Republic of Srpska, Bosnia and Herzegovina, and Serbia, see Table 3, are good examples of approaches taken to enhance the prescribing of pregabalin across all indications. The situation in Austria, Poland, and Slovenia will be closely monitored, see Tables 2 and 3, to see if they could also provide examples of potential ways forward to enhance the prescribing of pregabalin across all indications.
Lithuania, Norway and Sweden will also be closely monitored to see whether the originator company will be successful in limiting the prescribing of generic pregabalin in practice to epilepsy and GAD, with Lyrica prescribed and dispensed for neuropathic pain, see Table 2 and Appendix 2. Whether these countries will follow the examples of Bosnia and Herzegovina, Czech Republic, Estonia, Germany (historic), Republic of Srpska Bosnia and Herzegovina, and Serbia, see Table 3, once pregabalin is available and reimbursed remains to be seen.
It is interesting to note the different approaches taken by the originator company to the KVs in Germany initially compared with regional health authorities in England and Health Boards in Scotland, see Table 3. This acknowledges adherence to current stipulations of Social Code Book V serving as an example to other countries worried about such developments in the future, although this is now being challenged.
The introduction of reference priced systems with reimbursement typically just covering the costs of the lowest priced molecule is another way forward, given the extent of internal reference pricing across Europe once multiple sources of a product become available [1]. This works best if originator companies drop their prices to compete; alternatively, the situation is pre-empted as seen for instance in Spain, see Table 3. Alternatively, the price of the originator (brand name) is reduced over time despite the protestations of the originator manufacturer, as seen in South Korea, see Table 3. Difficulties could, potentially occur if reimbursement or substitution for one indication is not recommended, which could occur in Sweden for treatments for epilepsy, see Table 2. This has not currently been a problem in South Korea with multiple pregabalin packs available from different manufacturers, see Table 3. This situation could potentially reduce the attractiveness of the market to generics companies if originator (brand name) manufacturers are happy to drop their prices to those of generics to compete in the knowledge that patients may prefer to stay with the originator if copayments are the same in the absence of any substitution in pharmacies. This is, however, being resisted by the originator company in South Korea, see Table 3.
The developments surrounding Lyrica and generic pregabalin, including potential health authority activities to enhance the prescribing of generic pregabalin, will be closely monitored over the coming months. This will be combined with research on the resultant effect of prescribing and dispensing of pregabalin or Lyrica in practice. The objective will be to provide further guidance to health authorities with their increasing need to maximize savings from generics or biosimilars once they become available for at least one indication. This is essential to maintain the ideals of comprehensive and equitable healthcare especially in Europe.
We have documented different approaches to the availability of generic pregabalin, with countries such as Germany historically having measures in place to enhance the prescribing of generics once at least one indication is off patent. This contrasts with countries such as the UK where generic pregabalin can only be prescribed for some but not all indications. This appreciably reduces potential savings from the availability of generics, which is an increasing concern given ever growing pressures on available resources.
The situation in the UK will now be closely monitored following a recent court judgement post acceptance of the paper overturning the originator company’s patent for pregabalin for pain control; although, this is currently being challenged by the company [67].
We thank Ms Elina Asola for the current information regarding Finland, Ms Laura McCullagh and Ms Susan Spillane for the current information regarding Ireland, and Ms Marie-Camille Lenormand for information regarding France.
All authors wish to thank the English editing support provided by Ms Maysoon Delahunty, GaBI Journal Editor, for this manuscript.
There are no conflicts of interest from any author. However, the majority of authors are employed by ministries of health, health authorities and health insurance companies or are advisers to them. The content of the paper and the conclusions though are those of each author and may not necessarily reflect those of the organization that employs them.
This work was in part supported by grants from the Karolinska Institutet, Sweden.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Brian Godman1,2, BSc, PhD; Michael Wilcock3, MPharm; Andrew Martin4, MPharm; Scott Bryson2,5, MSc, MPH; Christoph Baumgärtel6, MD; Tomasz Bochenek7, MD, MPH, PhD; Winne de Bruyn8, BSc; Ljiljana Sović Brkičić9, MPharm; Marco D’Agata10, MSc; Antra Fogele11, PhD; Anna Coma Fusté12, MSc; Jessica Fraeyman13, PhD; Jurij Fürst14, MD; Kristina Garuoliene15,16, MD, PhD; Harald Herholz17, MD, MPH; Mikael Hoffmann18, MD, PhD; Sisira Jayathissa19, MBBS, MMedSc (Clin Epi), MD, FRCP (Lond, Edin), FRACP, FAFPHM, FNZCPHM, DClinEpi, DOPH, DHSM, MBS; Hye-Young Kwon20,21, BPharm, MPH, PhD; Irene Langner22, MA; Marija Kalaba23, MD; Eva Andersén Karlsson24,25, MD, PhD; Ott Laius26, PhD; Vanda Markovic-Pekovic27,28, PhD; Einar Magnusson29, MD; Stuart McTaggart30, MSc; Scott Metcalfe31, MBChB, DComH, FAFPHM (RACP), FNZCPHM; Hanne Bak Pedersen32, MD; Jutta Piessnegger33, PhD; Anne Marthe Ringerud34, MPharm; Gisbert W Selke22, BSc; Catherine Sermet35, MD; Krijn Schiffers36, BSc; Peter Skiold37, MSc; Juraj Slabý38, MD; Dominik Tomek39, Pharm Dr, PhD, MPH; Anita Viksna11, PhD; Agnes Vitry40, PhD; Corinne Zara12, MSc; Rickard E Malmström41, MD, PhD
1Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
2Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK
3Head of Prescribing Support Unit, Pharmacy Department, Royal Cornwall Hospitals NHS Trust, Truro, Cornwall TR1 3LJ, UK
4North West Commissioning Support Unit (NWCSU), Salford, Manchester M6 5FW, UK
5NHS Greater Glasgow & Clyde Prescribing Management Group, Glasgow, UK
6AGES Austrian Medicines and Medical Devices Agency and Austrian Federal Office for Safety in Health Care, 5 Traisengasse, AT-1200 Vienna, Austria
7Department of Drug Management, Faculty of Health Sciences, Jagiellonian University Medical College, Krakow, Poland
8Utrecht University, Utrecht, The Netherlands
9Croatian Health Insurance Fund, 37 Branimirova, Zagreb, Croatia
10Achmea Zorg and Health, 2 Handelsweg, NL-3707 NH Zeist, The Netherlands
11The National Health Service of Latvia, 31 k-3 Cēsuiela, LV-1012 Riga, Latvia
12Barcelona Health Region, Catalan Health Service, Barcelona, Spain
13Epidemiology and Social Medicine, Research Group Medical Sociology and Health Policy, University of Antwerp, Antwerp, Belgium
14Health Insurance Institute, Ljubljana, Slovenia
15Faculty of Medicine (Department of Pathology, Forensic Medicine and Pharmacology), Vilnius University, Vilnius, Lithuania
16State Medicines Control Agency, Vilnius, Lithuania
17Kassenärztliche Vereinigung Hessen, 15 Georg Voigt Strasse, DE-60325 Frankfurt am Main, Germany
18NEPI – Nätverk för läkemedelsepidemiologi, Sweden
19Department of Medicine, Hutt Valley DHB, Lower Hutt, Wellington, New Zealand
20Institute of Health and Environment, Seoul National University, Seoul, South Korea
21Department of Global Health and Population, Harvard School of Public Health, Boston, MA, USA
22Wissenschaftliches Institut der AOK (WIdO), 31 Rosenthaler Straße, DE-10178 Berlin, Germany
23Republic Institute for Health Insurance, Belgrade, Serbia
24,25Drug and Therapeutics Committee, Unit of Medicine Support, Public Healthcare Services, Stockholm County Council and Department of Clinical Science and Education, Karolinska Institutet, Södersjukhuset, Stockholm, Sweden
26State Agency of Medicines, Tartu, Estonia
27,28Faculty of Medicine, University of Banja Luka, Banja Luka, Republic Srpska, Bosnia and Herzegovina; Ministry of Health and Social Welfare, Banja Luka, Republic Srpska, Bosnia and Herzegovina
29Department of Health Services, Ministry of Health, Reykjavík, Iceland
30Public Health and Intelligence, NHS National Services Scotland, Edinburgh EH12 9EB, UK
31PHARMAC, 40 Mercer Street, Wellington 6011, New Zealand
32Health Technologies and Pharmaceuticals, Division of Health Systems and Public Health, WHO Regional Office for Europe, Copenhagen, Denmark
33Hauptverband der Österreichischen Sozialversicherungsträger, Vienna, Austria
34Section for Reimbursement, Department for Pharmacoeconomics, Norwegian Medicines Agency, 8 Sven Oftedals vei, NO-0950 Oslo, Norway
35IRDES, 10 rue Vauvenargues, FR-75018 Paris, France
36Erasmus University, Rotterdam, The Netherlands
37Dental and Pharmaceuticals Benefits Agency (TLV), PO Box 22520, 7 Flemingatan, SE-10422 Stockholm, Sweden
38State Institute for Drug Control, Czech Republic
39Department of Pharmacology, Faculty of Medicine, Slovak Medical University, Bratislava, Slovakia
40Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute, School of Pharmacy and Medical Sciences, University of South Australia, GPO Box 2471, Adelaide SA 5001, Australia
41Department of Medicine Solna, Karolinska Institutet, Clinical Pharmacology Karolinska University Hospital Solna, Stockholm, Sweden
References
1. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
2. Dylst P, Vulto A, Simoens S. Analysis of European policy towards generic medicines. Generics and Biosimilars Initiative Journal. 2014;3(1):34-5. doi:10.5639/gabij.2014.0301.011
3. Dylst P, Vulto A, Godman B, Simoens S. Generic medicines: solutions for a sustainable drug market? Appl Health Econ Health Policy. 2013;11(5):437-43.
4. Godman B, Abuelkhair M, Vitry A, Abdu S, Bennie M, Bishop I, et al. Payers endorse generics to enhance prescribing efficiency; impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2012.0102.017
5. Woerkom M, Piepenbrink H, Godman B, Metz Jd, Campbell S, Bennie M, et al. Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. J Comp Eff Res. 2012;1(6):527-38.
6. Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9(1):65-83.
7. Godman B, Bishop I, Finlayson AE, Campbell S, Kwon HY, Bennie M. Reforms and initiatives in Scotland in recent years to encourage the prescribing of generic drugs, their influence and implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):469-82.
8. Kaplan WA, Ritz LS, Vitello M, Wirtz VJ. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000-2010. Health Policy. 2012;106(3):211-24.
9. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
10. Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
11. Vogler S, Zimmermann N. How do regional sickness funds encourage more rational use of medicines, including the increase of generic uptake? A case study from Austria. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(2):65-75. doi:10.5639/gabij.2013.0202.027
12. All professionals urged to maximise use of generics. NHS Highland. the Pink One. 2014;110 extra (Special edition).
13. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300(21):2514-26.
14. Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70(5):605-21.
15. Corrao G, Soranna D, Merlino L, Mancia G. Similarity between generic and brand-name antihypertensive drugs for primary prevention of cardiovascular disease: evidence from a large population-based study. Eur J Clin Invest. 2014;44(10):933-9.
16. Veronin M. Should we have concerns with generic versus brand antimicrobial drugs? A review of issues. JPHSR. 2011;2(3):135-50.
17. Paton C. Generic clozapine: outcomes after switching formulations. Br J Psychiatry. 2006;189:184-5.
18. Araszkiewicz AA, Szabert K, Godman B, Wladysiuk M, Barbui C, Haycox A. Generic olanzapine: health authority opportunity or nightmare? Expert Rev Pharmacoecon Outcomes Res. 2008;8(6):549-55.
19. Baumgärtel C. Myths, questions, facts about generic drugs in the EU. Generics and Biosimilars Initiative Journal (GaBI). 2012;1(1):34-8. doi:10.5639/gabij.2012.0101.009
20. Ferner RE, Lenney W, Marriott JF. Controversy over generic substitution. BMJ. 2010;340:c2548.
21. Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing? Br J Clin Pharmacol. 2010;70(3):335-41.
22. Abuelkhair M, Abdu S, Godman B, Fahmy S, Malmstrom RE, Gustafsson LL. Imperative to consider multiple initiatives to maximize prescribing efficiency from generic availability: case history from Abu Dhabi. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):115-24.
23. Garuoliene K, Godman B, Gulbinovic J, Wettermark B, Haycox A. European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
24. Gemeinsamer Bundesausschuss. Anlage VII zum Abschnitt M der Arzneimittel-Richtlinie. Regelungen zur Austauschbarkeit von Arzneimitteln (aut idem). 10 Apr 2015 [homepage on the Internet]. 2015 May 22 [cited 2015 Jun 11]. Available from: https://www.g-ba.de/downloads/83-691-376/AM-RL-VII-Aut-idem_2015-04-10.pdf
25. Pfizer threatens pharmacists, doctors if they take its name in vain. 24 Dec 2014 [cited 2015 Jun 11]. Available from: http://boingboing.net/2014/12/24/pfizer-threatens-pharmacists.html
26. What a pain. Drugs and Therapeutics Bulletin. 2015;53(5):50.
27. Noonan KE. Patent Docs Biotech & Patent Law & News Blog [Internet]. The uncomfortable intersection between the practice of medicine and reality. 29 December 2014. [cited 2015 Jun 11]. Available from: http://www.patentdocs.org/2014/12/the-uncomfortable-intersection-between-the-practice-of-medicine-and-reality.html
28. Staton T. Pfizer adds another $325M to Neurontin settlement tally. Total? $945M. Fierce Pharma. 2 June 2014 [cited 2015 Jun 11]. Available from: http://www.fiercepharma.com/story/pfizer-adds-another-325 m-neurontin-settlement-tally-total-945 m/2014-06-02
29. Feeley J. Pfizer Neurontin class improperly denied, court says. Bloomberg Business. 3 April 2013. [cited 2015 Jun 11]. Available from: http://www.bloomberg.com/news/articles/2013-04-04/pfizer-neurontin-class-improperly-denied-appeals-court-says-1-
30. Lawrence J, Feeley J. Pfizer agrees to first Neurontin lawsuit settlement. Bloomberg Business. 2 April 2010. [cited 2015 Jun 11]. Available from: http://www.bloomberg.com/news/articles/2010-04-02/pfizer-agrees-to-first-settlement-of-a-neurontin-related-suicide-lawsuit
31. Newman M. Bitter pills for drug companies. BMJ. 2010;341:c5095
32. National Institute for Health and Care Excellence. Clinical Knowledge Summaries. Neuropathic pain – drug treatment. 1 February 2014 [homepage on the Internet]. [cited 2015 Jun 11] Available from: http://www.evidence.nhs.uk/document?ci=http%3a%2f%2fcks.nice.org.uk%2fneuropathic-pain-drug-treatment&returnUrl=Search%3fom%3d%5b%7b%22srn%22%3a%5b%22Clinical+Knowledge+Summaries+-+CKS%22%5d%7d%2c%7b%22toi%22%3a%5b%22Guidance%22%5d%7d%5d%26q%3dneuropathic%2bpain&q=neuropathic+pain
33. Scottish Medicines Consortium. Pregabalin 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg and 300 mg capsules (Lyrica®) No. (157/05). 9 April 2009 [homepage on the Internet]. 2009 Apr 22 [cited 2015 Jun 11]. Available from: https://www.scottishmedicines.org.uk/files/pregabalin__Lyrica__2nd_Resubmission_FINAL_April_2009_for_website.pdf
34. Spence D. Bad medicine: gabapentin and pregabalin. BMJ. 2013;347:f6747.
35. PBAC. Public Summary Document. Product: Pregabalin, capsules, 25 mg, 75 mg, 150 mg and 300 mg, Lyrica®. Sponsor: Pfizer Australia Pty Ltd. March 2012 [homepage on the Internet]. 2012 Jul 2 [cited 2015 Jun 11]. Available from: http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2012-03/pregabalin.pdf
36. Gustafsson LL, Wettermark B, Godman B, Andersen-Karlsson E, Bergman U, Hasselstrom J, et al. The ‘wise list’- a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108(4):224-33.
37. McCartney M. Margaret McCartney: Second use patents – why do we have to prescribe branded Lyrica for pain? BMJ. 2015;350:h2734.
38. GaBI Online – Generics and Biosimilars Initiative. United Kingdom – Policies and Regulations [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 11]. Available from: www.gabionline.net/Country-Focus/United-Kingdom/Policies-and-Legislation
39. MHRA. Antiepileptic drugs: new advice on switching between different manufacturers’ products for a particular drug.14 November 2013 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: https://www.gov.uk/drug-safety-update/antiepileptic-drugs-new-advice-on-switching-between-different-manufacturers-products-for-a-particular-drug http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON3367162013
40. England and Wales High Court (Patents Court) Decisions. Neutral Citation Number: [2015] EWHC 72 (Pat) – Case No: HC-2014-001795. Between Warner-Lambert Company, LLC (Claimant) and (1) Actavis Group PTC EHF; (2) Actavis UK LIMITED; (3) Caduceus Pharma Limited (Defendants) and (4) Highland Health Board. 21 January 2015 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.bailii.org/ew/cases/EWHC/Patents/2015/72.html
41. IPKAT [Internet]. No pain for Actavis – Warner-Lambert fail to stop launch of generic pregabalin. 21 January 2015. [cited 2015 Jun 11]. Available from: http://ipkitten.blogspot.co.uk/2015/01/no-pain-for-actavis-warner-lambert-fail.html
42. Bennie M, Bishop I, Godman B, Campbell S, Miranda J, Finlayson AE, et al. Are prescribing initiatives readily transferable across classes: the case of generic losartan in Scotland? Qual Prim Care. 2013;21(1):7-15.
43. Pharmacutical Services Negotiating Committee. Dispensing of Lyrica/Pregabalin. 28 January 2015 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://psnc.org.uk/our-news/dispensing-of-lyrica-pregabalin/
44. NHS Engand. Pubblications Gateway Reference 03188. Pregabalin – Frequently asked questions [homepage on the Internet]. 2015 Mar 6 [cited 2015 Jun 11]. Available from: http://www.england.nhs.uk/wp-content/uploads/2015/03/pregabalin-faqs.pdf
45. NHS England. Schedul e 1: The Pregabalin Guidance [homepage on the Internet]. 2015 Feb 27 [cited 2015 Jun 11]. Available from: http://www.palliativedrugs.com/download/Pregabalin_Guidance_NHS_England.pdf
46. JUVE. Blockbuster: Pfizer setzt mit Allen & Overy und Clifford Second-Medical-Use-Patent durch. 10 April 2015 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.juve.de/nachrichten/verfahren/2015/04/blockbuster-pfizer-setzt-mit-allen-overy-und-clifford-second-medical-use-patent-durch
47. Kurz und Bündig – Pregabalin-Generika – desinformierendes Ärzteanschreiben von Pfizer. arznei-telegramm. 2015;46:15-6.
48. Arbeitsgruppe Arzneimittelvereinbarung. Gemeinsame Information der KVWL und der Verbände der Krankenkassen in Westfalen-Lippe – Pregabalin-Generika seit dem 1. Dezember 2014 verfügbar – Hohes Einsparpotential [homepage on the Internet]. 2015 Dec 18 [cited 2015 Jun 11]. Available from: http://www.kvwl.de/arzt/verordnung/arzneimittel/info/agavm/pregabalin_agavm.pdf
49. Rahmenvertrag über die Arzneimittelversorgung nach § 129 Absatz 2 SGB V in der Fassung vom 15. Juni 2012 [homepage on the Internet]. 2015 Jun 19 [cited 2015 Jun 11]. Available from: http://www.gkv-spitzenverband.de/media/dokumente/krankenversicherung_1/arzneimittel/rahmenvertraege/apotheken/AM_20120615_S_RVtg_129_Abs2.pdf
50. Bundeministerium der Justiz und für Verbraucherschutz. Sozialgesetzbuch Fünftes Buch. §129 Rahmenvertrag über die Arzneimittelversorgung, Absatz 1, Satz 2, in der Fassung vom 22.12.2010 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.gesetze-im-internet.de/sgb_5/__129.html
51. Cacace M, Ettelt S, Mays N, Nolte E. Assessing quality in cross-country comparisons of health systems and policies: towards a set of generic quality criteria. Health Policy. 2013;112(1-2):156-62.
52. Baumgärtel C, Godman B, Malmström R, Andersen M, et al. What lessons can be learned from the launch of generic clopidogrel? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68. doi:10.5639/gabij.2012.0102.016
53. PHARMAC. Pregabalin – Ranked [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.pharmac.govt.nz/patients/ApplicationTracker? ProposalId=459
54. PHARMAC. PTAC meeting held 11 & 12 August 2011 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://pharmac.govt.nz/2011/08/02/2011%2008%20PTAC%20web%20 minutes.pdf
55. PHARMAC. Analgesic Subcommittee of PTAC meeting held 24 April 2012 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.pharmac.health.nz/assets/ptac-analgesic-subcommittee-minutes-2010-04-24.pdf
56. PHARMAC. Neurological Subcommittee of PTAC meeting held 27 August 2014 [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.pharmac.health.nz/assets/ptac-neurological-subcommittee-minutes-2014-08.pdf
57. PHARMAC. Pharmaceutical Management Agency – Purchasing medicines [homepage on the Internet]. 2011 Sep 15 [cited 2015 Jun 11]. Available from: https://www.pharmac.health.nz/assets/purchasing-medicines-information-sheet.pdf
58. Grandia L, Vulto A. Generics substitution in primary care: summary of the Dutch community pharmacies guidelines. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):102-3. doi:10.5639/gabij.2012.0102.021
59. TLV. TLV avskriver omprövning av Lyrica [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.tlv.se/lakemedel/omprovning-av-lakemedel/avslutade-omprovningar/Omprovning-av-Lyrica/
60. TLV. Efterlevnad subventionsbegränsning juli 2014 pregabalin (Lyrica) [homepage on the Internet]. [cited 2015 Jun 11]. Available from: http://www.tlv.se/Upload/Efterlevnad/efterlevnad_pregabalin_juli_2014.pdf
61. Matusewicz W, Godman B, Pedersen HB, Fürst J, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Research. 2015:15(5):755-8.
62. Pregabalin: prescribe and dispense generically. NHS Highland. the Pink One. 2014;111.
63. Article removed pending further discussion. NHS Highland. Available from: http://www.nhshighland.scot.nhs.uk/Publications/Documents/Newsletters/Pharmacy/The%20Pink%20One%20-%20Current%20Publication.pdf
64. Community Pharmacy Scotland. Supply of pregabalin [homepage on the Internet]. 2015 Feb 18 [cited 2015 Jun 11]. Available from: http://www.communitypharmacyscotland.org.uk/media/86593/Dispensing-of-Lyrica.pdf
65. NHS England. Public Health England. Advice for prescribers on the risk of the misuse of pregabalin and gabapentin [homepage on the Internet]. 2014 Aug 10 [cited 2015 Jun 11]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/385791/PHE-NHS_England_pregabalin_and_gabapentin_advice_Dec_2014.pdf
66. Godman B, Malmstrom RE, Diogene E, Gray A, Jayathissa S, Timoney A, et al. Are new models needed to optimize the utilization of new medicines to sustain healthcare systems? Expert Rev Clin Pharmacol. 2015;8(1):77-94.
67. Kmietowicz Z. Pfizer loses UK patent for blockbuster pain drug after threats to doctors. BMJ. 2015;351:h4918.
|
Author for correspondence: Brian Godman, BSc, PhD, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/generic-pregabalin-current-situation-and-implications-for-health-authorities-generics-and-biosimilars-manufacturers-in-the-future.html
Author byline as per print journal: Ljiljana Sović Brkičić, MPharm; Brian Godman, BSc, PhD; Martina Bogut, BSc; Miron Sršen, MD; Hye-Young Kwon, BPharm, MPH, PhD; Winnie de Bruyn, BSc; Tonko Tabain, MD
|
Introduction: Croatia has introduced a number of reforms to contain pharmaceutical expenditure whilst increasing access to new medicines. These include new regulations and new ordinances in 2013 including the pricing of new medicines and lowering the price of generics. |
Submitted: 19 January 2015; Revised: 24 April 2015; Accepted: 27 April 2015; Published online first: 11 May 2015
Rising pharmaceutical expenditure is causing concern among countries, with expenditure rising by more than 50% in real terms during the past decade among OECD countries [1, 2]. These concerns have resulted in multiple reforms and initiatives across Europe, which includes regulations regarding prices, reimbursement and utilization of medicines [2, 3]. Initiatives for established medicines include measures to obtain low prices for generics as well as encourage their prescribing [3–9]. This includes both internal reference pricing (IRP) systems and external reference pricing (ERP) [9–13], with IRP currently utilized among 20 or more EU Member States and ERP among 24 EU Member States [9, 12]. Croatia is no different and has introduced a number of reforms in recent years to reduce debt levels, add new medicines to the reimbursement list and improve the quality of care [14–17]. Measures include restricting medicines to second line, with follow-up by physicians working for the Croatian Health Insurance Fund (CHIF) if abuse is suspected [18], as well as strict control of pharmaceutical company activities, with adherence enhanced through financial penalties [14, 15]. In addition, regulations for lowering the prices of successive generics for a given molecule are in place [14, 18].
However, there is a continual need to conserve resources, as well as a continuing need to encourage the reimbursement of new valued medicines, given the level of unmet need, ageing populations and continued high unemployment affecting CHIF revenues [19, 20]. This led to the development of a new ordinance for the pricing of both new and established medicines including biosimilars. The old ordinance was published in the Official Gazette 155/2009, and its amendment published in the Official Gazette 22/2010, while the new ordinance was published in the Official Gazette 83/2013 and its amendment in the Official Gazette 12/2014 and Official Gazette 69/2014 [19–24]. There is a recognized need to evaluate the influence of the new ordinances on potential savings, and use the findings to plan further pertinent measures.
The ordinance establishing the criteria for the inclusion of medicinal products onto the basic and supplementary Reimbursement Lists of the CHIF has been enacted since 2013 (Official Gazette 83/2013) [20]. The decision on accepting or rejecting new medicines for reimbursement is undertaken by the Committee for Drugs and Medicinal Products within the Administrative Council of the CHIF following a recommendation by the Commission for Drugs, which consists of 13 members who are all experts in their specific disease area.
Key criteria for assessing the value of new medicines in Croatia, and their potential prices, include improved outcomes versus current standards including improved quality of life and/or reduced adverse effects, more ‘user-friendly’ formulations improving compliance as well as improved overall efficiency [14, 15]. In addition, whether this is a medicine where no treatment has previously existed; alternatively, a replacement treatment. There is also the potential for price reductions or price: volume agreements, including pay-backs or cross-product agreements, for new active substances with budget impact analyses requested for new medicines based on best practice and defined by the ordinance [14, 15, 17, 20].
The prices of established as well as new medicines are regulated by ordinances including biosimilars, vaccines and small molecules [19, 20]. The ordinances also describe the procedure of calculating annual drug pricing (external reference pricing, ERP) as well as the method for setting reference prices of medicines and reference price systems (internal reference pricing, IRP). ERP is a process in which the prices of medicines are compared with prices of medicines in comparable countries (example contained in Table 3). IRP is based on the Anatomical Therapeutic Chemical (ATC) classification. This can be at ATC Level III (pharmacologic group, e.g. lipid modifying agents), Level IV (pharmacologic class, e.g. HMG CoA reductase inhibitors) or Level V (individual molecule) [14, 17, 25]. IRP is a process in which CHIF established the reference prices of medicines, which are the reimbursed prices (Example contained in Table 4). CHIF covers the reference price, with patients covering any additional costs themselves for a more expensive product out-of-pocket.
The reimbursed price on the two lists (basic and supplementary) is the ex-factory price combined with wholesale margins and other taxes up to 8.5%, which is wholesale price (WP) and tax (VAT) at 5%, as well as a pharmacy mark-up (fee) which is constant per package of medicines. Prices for non-reimbursed medicines including over-the-counter medicines are typically determined by market demand. Prices include WP, taxes (5% and 25%) and pharmaceutical margins (up to 35%).
The ordinances conform to the Directives of the European Commission regarding the pricing of medicines. According to these directives, each country determines the price of their medicines on the basis of a self-selected model, which must be clear and transparent, and must be implemented within set deadlines and in compliance with the directives [26]. The basic list of drugs, which are fully reimbursed, and the supplementary list of drugs, with CHIF covering the reimbursed part with the patient covering the remainder, are the final results of the current procedures in 2013 [27, 28]. The basic list also includes expensive medicines that are funded out of different budgets. Drug lists are constantly changing as new drugs are included, indications are revised and established medicines removed [27–29].
In Croatia, the share of public expenditures for health care is currently 6.6% of GDP [30, 31], with 14.6% spent on pharmaceuticals in 2012 [31]. This is down from 17% in 2007 [31]. The various reforms in recent years have resulted in similar financial expenditure on prescription medicines in 2012 compared with 2008, i.e. Kunas 3,303 billion in 2012 (Euros 433 million) versus Kunas 3,392 billion (Euros 445 million) in 2008. In addition between 2009 and 2011, 85 new medicines were added to the reimbursement list [30]. Overall, 3,044 packages of medicines were included in the basic drug list in 2012 and 390 drugs on supplementary list. This compares with 2,047 packages of medicines in the basic list and 262 in the supplementary list in 2008.
The objectives of this paper are to: 1) report on the new ordinances including changes in the reference price systems and compare these with the previous ordinances; 2) compare potential savings from the changes in the reference price system (internal reference pricing, IRP) at ATC Level III to V (current system) to just ATC Level V for comparative purposes among the 54 current reference price groups as a result of the recent reforms; 3) suggest additional measures for consideration by CHIF in the future as CHIF strives to continue to provide comprehensive and equitable healthcare.
We are not aware of many publications that have assessed the impact of changes to their ERP and IRP systems, although authors have calculated potential savings through converging prices to the average [12]. Consequently, we hope the findings and their implications will be of interest to the authorities in Croatia as well as other European countries that use reference pricing systems since we are aware there are some concerns with reference pricing initiatives [10, 17]. We will discuss these in the context of the findings in Croatia to stimulate further debate in this growing area of interest.
This includes a narrative review of the reforms comparing the old ordinances (2009) with the new ordinances (2013) among the co-authors (principally LSB), including the regulations surrounding:
a. Placing new medicines on the list either as replacements for existing established medicines or entirely new treatments where none have existed before. This will be compared and contrasted with the old ordinance.
b. Placing new generic medicines onto the drug lists, as well as comparing the old and new ordinances.
c. The model for calculating the ERP for medicines and the implications, see Table 3.
d. Internal pricing using a reference price system. We will describe the model of the IRP as well as the projected potential savings based on expenditure of medicines in the various reference price groups in 2012, see Table 4.
For ERP, this included comparing potential savings with the old and new ordinances.
The calculations of potential savings from the changes in the IRP system for the 54 product groups are included in Box 1.
The potential savings arising from all the changes in the ordinances will also be calculated to provide a basis for any pertinent additional future reforms. The analytic methods applied in the various situations are descriptive epidemiological methods, primarily involving a comparative analysis of pricing models of medicines under the old and new ordinances.
The quality of the data is assured by regular auditing of the CHIF database.
Suggestions for potential additional measures and reforms that CHIF could consider introducing in the future will be based on the considerable experience of the co-authors working with health authorities from different countries in analysing health reform policies.
This will be divided into four sections starting with placing new medicines placed onto the reimbursed list including new generics.
A. Pricing of new medicines or new treatments including new generics
The principal difference between the old and new ordinances is the pricing of biosimilars as well as new hospital medicines, see Table 1. Hospital medicines are now included where there is an added health benefit compared with existing standards as there have been problems with their pricing in the past in the implementation and finalization proceedings. The list price in the new ordinance for new active substances demonstrating additional health benefit versus existing standards is now increased to 100% of their calculated prices, up from 90% of the comparable medicine. There is a 10% patient copayment for ambulatory care medicines but not hospital medicines, see Table 1.
For new medicines or new active treatments where no treatment has existed before for the particular disease, the price is calculated based on ERP, see Table 2 as well as a % of the Average Comparable Price (ACP), see Table 1, based on prices of similar treatments in comparable countries, see Table 2.
It is envisaged the order of the countries used for ERP, see Table 2, alongside changes from the factors relating to retail versus wholesale prices, will affect subsequent reimbursed prices for new medicines in Croatia. This is because the average price of the package of medicines in comparable countries (without tax and margins) is translated into the unit price (in Kunas) for that country and then into the price of the package of the drug that will be available in Croatia. After the translation, the ACP of the drug is calculated. The ACP is calculated as the average price of the same drug (same generic name, and the same or similar form and packaging) in the three chosen countries starting with Italy, Slovenia and Czech Republic, according to the new order established by the ordinance, see Table 2. In cases where there is no comparative price for the new medicine in one country, then the average price of the medicine from the next country in the list of chosen countries is included and so on, see Table 2.
As seen in Table 1, the price calculations for the first and subsequent generics for small molecules are similar between the old and new ordinances. The main difference is that biosimilars are now included to address this anomaly, with the price of the first biosimilar at 85% of the originator price. The inclusion of biosimilars should accelerate savings as more biosimilars are launched for existing molecules and more biologicals lose their patents given the current high prices for biological medicines [32–38].
B. Pricing of medicines under external reference pricing
The principal differences in the ordinances for the ERP of existing medicines and new medicines where no medicine has existed before is the order of the reference price countries for calculating external reference prices, see Table 2.
ERP of established medicines is carried out once a year to make sure that the prices of established medicines in Croatia are not higher than the chosen European countries. ERP for established medicines used to take place the first Monday in February each year [20], but will now take place on the first Monday in March [21]. If the price of the medicine in Croatia is found to be higher than the ACP price, its price is subsequently reduced to the level of the ACP.
The sources for external pricing data are similar, i.e. official data from the countries included in the ordinances, however, there is now greater reliance on web sources than publications, see Table 2. Data sources include:
• Old ordinance: Italy – Informatore Farmaceutico; France – Vidal; Slovenia – Register; Spain – Catalogo de Medicamentos; Czech Republic – www.vzp.cz
• New ordinance: Italy – www.Codifa.it; Slovenia – http://www.jazmp.si; Czech Republic – http://www.sukl.cz/; Spain – Catalogo de Medicamentos: latest electronic version; France – Vidal Expert: latest electronic version
Other changes include greater interaction with the legal persons and their representatives in each country, which are involved in the registration and/or pricing of medicines during the reference pricing process.
There have also been changes to some of the factors for the recalculation from retail to wholesale prices to remove taxes and margins from the included countries and to enable comparisons based on WPs. The presented factors for the recalculation of the reference prices for the comparable countries, published table as a part of the ordinances, are included in Table 2.
Under the new ordinances, prices remain at 100% of the ACP of the medicine under the various regulations for new and established medicines including generics and biosimilars, see Tables 1 and 2. Consequently, the calculation of the prices of both new and established medicines under the new ordinance appears applicable and acceptable to all key stakeholder groups. This was not the case with the old ordinances, especially if Croatia was a reference price country, as under the old ordinance prices were reduced by 10% and 35% of the ACP, i.e. prices were at 90% if patent protected and 65% if no patent protection of the ACP, see Table 2. Having said this, the order of countries for ERP has changed potentially impacting on prices in Croatia along with changes in the factors involved, see Table 2. In addition, the wording of the ordinances has changed, see Table 2. 100% under the new ordinances for both originator and generic products enables fair market competition based on ACPs.
Table 3 provides an example of the impact of the change in the ERP policy for omeprazole, with the change in the factors for calculating ACPs based on the change in factors from retail to wholesale prices as well as changes in the order of the countries, see Table 2.
C. Internal reference pricing system
The internal reference pricing system (IRP) has been introduced since it can happen on the reimbursed list that there are the same medicines with different prices. This is because new medicines can be placed on the list which contain the same INN, same dosage and packaging but with different prices due for instance to successive generics necessarily being priced 10% lower than the previous one for reimbursement, see Table 1. Similarly, new single-sourced products (ATC Level III to V) can be placed on the reimbursement list at potentially lower prices. Similar to ERP, IRP takes place once a year once ERP has been performed. If the prices of the same medicine differ, their prices are subsequently reduced to the level of the reference price or lower.
Prices of the originators are subsequently subject to IRP based on the lowest priced molecule in the class (ATC Level III to V) with a market share of 5% or more of the total market by volume. Should the authorization holder not accept the proposed price, i.e. want to keep the old price, the medicine is placed on the supplementary list with CHIF paying the reference price with patients covering any additional price themselves out-of-pocket.
To illustrate this, a comparison has been performed, see Table 4, building on the methodology described in Box 1. Reference prices are determined in relation to the unit price of the drug, or at the price of the package for the drug, or the amount of active compound in a unit form of the medicine based on similar defined daily doses (DDDs) [25]. We are aware the World Health Organization (WHO) does not recommend the use of DDDs to compare prices of medicines. However, we are not aware of another appropriate comparator for determining (comparison) of prices especially if dosage forms and pack sizes vary. Determining reference prices of medicines by ATC Level III to V helps ensure similar prices are paid for the same molecule as well as for medicines that have the same or similar therapeutic effect. The reference price of a medicine requiring a prescription is determined by searching for the medicine with the lowest price within the pertinent reference group, which in the previous year had a market share of at least 5% [14]. We are also aware the reimbursement list may contain medicines that have not achieved a market share of 5%; however, their price cannot be used as reference price.
The reference groups (currently 54) for IRP are determined by the Committee for Medicinal Products of the Ministry of Health. If the marketing authorization holder for the medicine, or its authorized representative, accepts the proposed reference price, their medicines will be placed on the basic list of drugs. As mentioned, if the authorization holder (originator or branded generic) rejects the proposed reference price, or keeps the old price, the medicine is placed on the supplementary list of medicines and the CHIF just pays the reference price for the particular medicine. Any difference in the price is subsequently paid by the patient out-of-pocket. The authorization holder (legal persons and their representatives) may also propose a price of their medicine that is lower than the current reference price to enhance its market share.
Table 4 provides an example of the model for the IRP system (ATC Level III to V) for the proton pump inhibitors (PPIs) in 2012.
D. Potential savings with the new ordinances
Calculations undertaken before the new ordinances were implemented regarding ERP believed the new ordinances, with changes in the order of the reference price countries, see Table 2, would lower prices by an average of 8% to 10%. This is less than the potential savings of 10% to 35% under the old ordinance with prices lowered by 65% to 90% of the ACP, see Table 2. However, the documented price reductions under the old ordinance were seen as large and potentially restrictive. The changes in the new ordinances to 100% of comparative prices, see Table 1, are seen as more acceptable to key stakeholder groups especially if Croatia is a reference priced country.
The overall projections for potential savings are based on changes in the ERP system with the new ordinances, incorporating all medicines in the basic and supplementary list including generics and biosimilars, factors concerned with wholesale and retail prices as well as changes in the order of the reference countries, see Tables 1 and 2, described in the Methodology section. This does not apply to new medicines where none has existed before to treat a given disease as these costs will be in addition. There are separate projections based on changes in the IRP system, including medicines placed in the 54 reference groups. As mentioned, IRP in Croatia is determined at ATC Level III to V. Consequently, this was the principal model used to calculate potential savings for the new ordinances in the current reference groups. Potential savings were compared to just concentrating at ATC Level V when determining potential IRP for comparative purposes.
Based on 2011 consumption and projected savings per package in 2012 outlined in the Methodology section, projections showed possible savings of Kunas 318.4 million for CHIF for the 54 reference groups, see Table 5. This represents savings of 9.64% for reimbursed drugs based on projected 2012 consumption figures. Setting the reference price of medicines (IRP) at the ATC Level V rather than III to V yielded potential savings of Kunas 254.45 million, i.e. 7.7% of total CHIF expenditure on ambulatory care medicines in 2012 [34]. Table 5 shows projected savings for each of the 54 reference groups (ATC Level III to V). 2,028 packages of different medicines were included in the 54 reference price groups in 2012, forming the basis of the calculated savings.
Projected savings from the new ordinances for CHIF for medicines on the current lists will grow as more generic medicines are launched as well as more biosimilars for the same medicine and for new biological medicines losing their patents.
Different European countries have used different models and approaches for determining the price of new and established medicines including generics. This includes both IRP and ERP, with differences in ERP in terms of the number of countries chosen, their sequence, methods for establishing reference prices as well as time frames for their review [10, 17, 39–42].
This paper demonstrates that changes in the reference pricing system can lead to considerable differences in overall reimbursed expenditure. There have been concerns that countries with small populations cannot obtain low prices for medicines [40]. However, this paper demonstrates that a European country with a smaller population, i.e. 4.27 million inhabitants in 2012 year, was active with introducing a variety of measures to help control pharmaceutical expenditure whilst increasing access to new medicines. This includes changes in reference price systems and the pricing of medicines losing their patent.
The calculated savings from the changes in the ERP system, including the order of chosen countries, see Table 2, were estimated at an average of 8% to 10%. The calculated savings from the changes in the IRP systems were estimated at Kunas 318.4 million among the 54 reference groups, see Table 5, i.e. 9.64% of reimbursed drug expenditure in 2012, reduced to Kunas 254.45 million, i.e. 7.7% of total CHIF expenditure, if the IRP is just based on ATC Level V [34]. These findings endorse CHIF’s decision to use ATC Level III to V rather than just ATC V for IRP in the new ordinances. This provides guidance to other countries using reference pricing particularly once generics become available in a class to help control pharmaceutical expenditure. This difference is also a potential way to address concerns regarding the lack of transparency in the prices of reference priced countries.
We are aware of a number of controversies surrounding ERP. This includes the fact that prices of medicines in countries may not reflect actual prices [10, 12, 43, 44]. In addition, pharmaceutical companies may preferentially launch their new medicines initially in traditionally higher price countries thereby potentially increasing the prices in the remaining countries that directly or indirectly reference them [12]. Thirdly, price reductions in one reference country may not automatically apply to other reference countries unless there are mechanisms to rapidly assess this. As a result, reduce potential savings [12]. Finally, pharmaceutical companies could potentially withhold launching their new medicines in lower priced countries as this may adversely affect overall profitability. However, the initial reforms in Croatia resulted in 85 new medicines being added to the reimbursement list between 2009 and 2011 coupled with a deficit reduction [14, 30]. This was up from 47 new medicines between July 2009 and 2010, with 13 new medicines added to the list of expensive hospital products [15]. In addition, we believe the changes in the new ordinance, see Tables 1 and 2, should be beneficial to all key stakeholder groups with prices of both patented and multiple sourced medicines remaining at 100% as opposed to 65% to 90% of ACP. Whilst potentially resulting in lower savings for CHIF, this should enhance the attractiveness of this ordinance to key stakeholder groups, addressing some of the concerns that companies will not launch their new medicines in lower priced countries. The new ordinances with no automatic price reductions for new medicines, see Tables 1 and 2, should also be beneficial to pharmaceutical companies enhancing their desire to launch new medicines in Croatia. As mentioned, lower prices for established medicines including generics and biosimilars should be beneficial to patients, reducing their copayment levels as well as creating headroom for new medicines.
We are also aware that there are controversies surrounding the selection of countries for ERP, although most countries appear to reference those of similar income levels [10]. In this respect, we believe the change in the order of countries brings Croatia in line with other European countries, i.e. higher income countries tend to include higher income countries in their basket whereas lower income countries tend to reference lower income countries [10]. The use of five countries is also similar to other European countries, who tend to have less than 10 countries in their reference baskets [10]. Wholesale prices should also be more uniform that pharmacy prices as there can be considerable differences in pharmacy remuneration and taxes between countries [10]. As a result, endorsing the new approach, see Tables 1 and 2.
We are aware that some European countries review their prices more regularly than Croatia to improve transparency [9, 11]. Applying this approach in Croatia could potentially lead to greater savings than those achieved by the recent changes in the ordinances, see Tables 1, 2, and 5. However, instigating a greater number of internal and external pricing reviews would need new procedures and workflows as well as consensus in how this can be achieved. In addition, an assessment of the implications for all key stakeholder groups including wholesalers, pharmacies, marketing authorization holders and others. Alongside this, the necessity to have transparent information systems that will continually monitor prices of medicines in the ERP countries as well as possible changes to legal entities. The increased costs would impact on the extent of any potential savings. Having said this, this possibility should not be discarded if further savings are needed in Croatia in the future.
Finally, we are aware that the new ordinance has not discussed potential initiatives to improve the quality of prescribing apart from prescribing restrictions for certain medicines, e.g. angiotensin receptor blockers and curbing pharmaceutical company marketing activities [14, 18]. In addition, the instigation of e-prescribing from January 2011 is making medicines more accessible without patients visiting their physician. Possible measures could include initiatives to reduce adverse drug reactions (ADRs) and drug interactions including decision support systems in conjunction with e-prescribing [45, 46]. Treating ADRs can be costly to health authorities as well as adversely affecting the health of patients. Published studies have shown that ADRs add to the costs of health care through increasing hospital admissions and other costs [47–49]. For example, the average treatment costs in Germany were estimated at approximately Euros 2,250/ADR, equating to Euros 434 million per year [50], with the cost of drug-related morbidity and mortality exceeding US$177.4 billion in the US in 2000 [51]. This is despite the proclaimed goal of the authorities in Croatia to introduce and implement for instance external evaluation of the quality of healthcare institutions [31].
The strength of these findings is based on the fact that the ordinances and findings are based on CHIF data, which is regularly audited. The weakness is the fact that these are projections. We will continue to monitor the situation and provide feedback to CHIF if further ordinances are needed.
Potential ways forward in addition to potential price cuts to help Croatia stay within agreed pharmaceutical expenditure [52] could include greater education of patients concerning the medicines they receive. As a result, reduce unnecessary requests for medicines as well as improve compliance, which is a concern in patients with chronic asymptomatic diseases [53, 54]. Other potential initiatives could include the instigation of active regional drugs and therapeutic committees deciding which medicines to use to treat common diseases in ambulatory care, building on the current reimbursement list. This is because there are concerns with the evidence base of the medicines included in the current reimbursement list [55], as well as typically physicians only knowing a relatively limited number of medicines well. This was the philosophy of the Stockholm Metropolitan Healthcare Region in Sweden where there has been a tradition of advocating ‘each recommended medicine should be of high value to the patient’ [56, 57]. Since 2000, approximately 200 medicines have been selected for common diseases in ambulatory care [56]. Respected specialists, working jointly with clinical pharmacologists, pharmacists and general practitioners in over 20 expert groups, suggest which medicines should be selected and included in the ‘Wise List’ [56, 58], which is then widely communicated and disseminated throughout Stockholm [56, 57]. Physician adherence to the voluntary ‘Wise List’ has increased during the past 10 years, now reaching 87% of all prescriptions, enhanced by physician trust in the ‘Wise List’ with its robust methodologies [56, 58].The ‘Wise List’ is now being translated into other languages to provide a stimulus to introducing such initiatives [59].
Concurrent with this, there is also likely to be increasing scrutiny over the value of new medicines where there is currently no existing treatment in Croatia for comparative purposes. This recognizes the appreciable number of new medicines in development, especially new biological medicines which are currently often priced at between US$100,000–US$400,000 (Euros 74,000–296,000) per patient per course or per year [2, 35, 36, 38, 60–62].
In conclusion, we have described the similarities and changes to the ordinance in Croatia for both new and established medicines including generics and biosimilars and the implications. In addition, the implications for savings through reordering the list of reference priced countries (ERP) as well as the subsequent implications for savings with IRP at ATC Level III to V versus ATC Level V alone. We hope the findings will be of interest to other European countries. In addition, demonstrate that European countries with smaller populations can be active with introducing a variety of measures when needed. This is in the best interests of all key stakeholder groups.
All authors approved the final version of the paper:
• LSB was the principal initiator of the paper, provided input regarding ongoing reforms in Croatia, was involved in the calculations of potential savings, wrote the first draft and critiqued subsequent drafts
• BBG critiqued the first draft based on his considerable experience in working with health authorities from different countries in analysing health reform policies. This first draft is used as a basis to develop the paper for submission and publication
• MB was heavily involved in the study design, provided input into ongoing reforms in Croatia, helped produce the first draft and critiqued subsequent drafts
• MS was principally involved with undertaking the calculations to ascertain potential savings from the different ordinances
H-YK and WB helped critique subsequent drafts based on their experiences
• TT was involved with the study design as well as critiqued the first and subsequent drafts
Competing interest: There are no conflicts of interest from any author. However, Ms Ljiljana Sović Brkićić is employed by CHIF, and Ms Martina Bogut is employed by the Ministry of Health Croatia.
This writing of this paper was in part supported by grants from the Karolinska Institutet, Sweden.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ljiljana Sović Brkićić1, MPharm
Brian Godman2,3, BSc, PhD
Martina Bogut4, BSc
Miron Sršen5, MD
Hye-Young Kwon6,7, BPharm, MPH, PhD
Winnie de Bruyn8, BSc
Tonko Tabain9, MD
1Croatian Health Insurance Fund, 37 Branimirova, HR-10000 Zagreb, Croatia
2Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, SE-14186, Stockholm, Sweden
3Strathclyde Institute of Pharmacy and Biomedical Sciences, Strathclyde University, Glasgow, UK
4Ministry of Health, 200a Ksaver, HR-10000 Zagreb, Croatia
5Sygin istraživanje i razvoj Ltd, 3 Trg žrtava fašizma, HR-10000 Zagreb, Croatia
6Institute of Health and Environment, Seoul National University, Seoul, South Korea
7Department of Global Health and Population, Harvard School of Public Health, Boston, MA, USA
8Department of Pharmaceutical Sciences, Utrecht University, Utrecht, The Netherlands
9University of Zagreb, School of Medicine, Zagreb, Croatia
Authors are responsible for English language editing of this manuscript.
References
1. OECD. OECD, Health at a Glance 2011: OECD Indicators. Pharmaceutical expenditure OECD Publishing [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://dx.doi.org/10.1787/health_glance-2011-en
2. WHO. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.euro.who.int/en/health-topics/Health-systems/medicines/publications2/2015/access-to-new-medicines-in-europe-technical-review-of-policy-initiatives-and-opportunities-for-collaboration-and-research
3. Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
4. Moon JC, Godman B, Petzold M, Alvarez-Madrazo S, Bennett K, Bishop I, et al. Different initiatives across Europe to enhance losartan utilisation post generics: impact and implications. Front Pharmacol. 2014;5:219.
5. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
6. Dylst P, Simoens S. Does the market share of generic medicines influence the price level?: a European analysis. Pharmacoeconomics. 2011;29(10):875-82.
7. Godman B, Abuelkhair M, Vitry A, Abdu S, Bennie M, Bishop I, et al. Payers endorse generics to enhance prescribing efficiency; impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2012.0102.017
8. Vogler S, Zimmermann N. How do regional sickness funds encourage more rational use of medicines, including the increase of generic uptake? A case study from Austria. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(2):65-75. doi:10.5639/gabij.2013.0202.027
9. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
10. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe: a descriptive overview. Health Policy. 2012;104(1):50-60.
11. World Health Organization. Espin J, Rovira J, Olry de Labry A. Working Paper 1: External reference pricing. WHO/HAI Project on medicine prices and availability. Review series on pharmaceutical pricing policies and interventions. May 2011 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.haiweb.org/medicineprices/05062011/ERP%20final%20May2011.pdf
12. European Commission. European Economy. Carone G, Schwierz C, Xavie A. Cost-containment policies in public pharmaceutical spending in the EU. Economic Papers 461. September 2012 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://ec.europa.eu/economy_finance/publications/economic_paper/2012/pdf/ecp_461_en.pdf
13. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
14. Brkicic LS, Godman B, Voncina L, Sovic S, Relja M. Initiatives to improve prescribing efficiency for drugs to treat Parkinson’s disease in Croatia: influence and future directions. Expert Rev Pharmacoecon Outcomes Res. 2012;12(3):373-84.
15. Voncina L, Strizrep T. Croatia: 2009/2010 pharmaceutical pricing and reimbursement reform. Eurohealth. 2011;16(4):20-2.
16. Godman B, Bennie M, Baumgärtel C, Sović Brkićić L, Burkhardt T, Fürst J, et al. Essential to increase the use of generics in Europe to maintain comprehensive healthcare? Farmeconomia: Health Economics and Therapeutic Pathways. 2012;13(Suppl 3):5-20.
17. Vogler S, Habl C, Bogut M, Voncina L. Comparing pharmaceutical pricing and reimbursement policies in Croatia to the European Union Member States. Croat Med J. 2011;52(2):183-97.
18. Voncina L, Strizrep T, Godman B, Bennie M, Bishop I, Campbell S, et al. Influence of demand-side measures to enhance renin-angiotensin prescribing efficiency in Europe: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):469-79.
19. Ministry of Health. Ordinance establishing the criteria for wholesale pricing of medicinal products and the manner of reporting wholesale prices. Official Gazette 155/2009.
20. Ministry of Health. Ordinance amending the corrections of Ordinance establishing the criteria for wholesale pricing of medicinal products and the manner of reporting wholesale prices. Official Gazette 22/2010. 2010.
21. Ministry of Health Croatia. Ordinance establishing the criteria for wholesale pricing of medicinal products and the manner of reporting wholesale prices. Official Gazette 83/2013 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.hzzo.hr/zdravstveni-sustav-rh/pravilnik-o-mjerilima-za-stavljanje-lijekova-na-osnovnu-i-dopunsku-listu
22. Ministry of Health Croatia. Ordinance establishing the criteria for the inclusion of medicinal products to the basic and supplementary Reimbursement Lists of the Croatian Health Insurance Fund. Official Gazette 83/2013 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://narodne-novine.nn.hr/default.aspx
23. Ministry of Health. Ordinance establishing the criteria for wholesale pricing of medicinal products and the manner of reporting wholesale prices. Official Gazette 12/2014. 2014.
24. Ministry of Health. Ordinance establishing the criteria for wholesale pricing of medicinal products and the manner of reporting wholesale prices. Official Gazette 69/2014. 2014.
25. WHO Collaborating Centre for Drug Statistics Methodology. ATC / DDD index 2011. WHO, Oslo. 2013 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.whocc.no/atc_ddd_index/
26. European Commission. European Commission Directive. Council Directive 89/105/EEC of 21 December 1988 relating to the transparency of measures regulating the pricing of medicinaI products for human use and their inclusion in the scope of national health insurance systems [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://ec.europa.eu/health/files/eudralex/vol-1/dir_1989_105/dir_1989_105_en.pdf
27. Croatian Health Insurance Fund. Supplementary Reimbursement List of the Croatian Health Insurance Fund. Official Gazette 9/2014.
28. Croatian Health Insurance Fund Basic Reimbursement List of the Croatian Health Insurance Fund. Official Gazette 9/2014.
29. Croatian Health Insurance Fund. Objavljene liste lijekova [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.hzzo.hr/zdravstveni-sustav-rh/trazilica-za-lijekove-s-vazecih-lista2014
30. Ministry of Health of the Republic of Croatia. National Healthcare Strategy 2012–2020 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.zdravlje.hr/programi_i_projekti/nacionalne_strategije/nacionalna_strategija_zdravstva.
31. Džakula A, Pavić N, Lonćarek K, Sekelj-Kauzlarić K. Croatia: health systems review. Health Systems in Transition. 2014;16(3).
32. Godman B, Malmstrom RE, Diogene E, Jayathissa S, McTaggart S, Cars T, et al. Dabigatran – a continuing exemplar case history demonstrating the need for comprehensive models to optimize the utilization of new drugs. Front Pharmacol. 2014;5:109.
33. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198-206.
34. Cohen D, Raftery J. Paying twice: questions over high cost of cystic fibrosis drug developed with charitable funding. BMJ. 2014;348:g1445.
35. Kmietowicz Z. NICE says drug for metastatic breast cancer is unaffordable for NHS. BMJ. 2014;348:g2888.
36. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439-42.
37. Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium—the just price. J Clin Oncol. 2013;31(28):3600-4.
38. Cohen P, Felix A. Are payers treating orphan drugs differently? Journal of Market Access & Health Policy. 2014;2:23513.
39. Leopold C, Mantel-Teeuwisse AK, Seyfang L, Vogler S, de Joncheere K, Laing RO, et al. Impact of external price referencing on medicine prices – a price comparison among 14 European countries. South Med Rev. 2012;5(2):34-41.
40. Godman B, Campbell S, Suh HS, Finlayson A, Bennie M, Gustafsson L. Ongoing measures to enhance prescribing efficiency across Europe: implications for other countries. J Health Tech Assess. 2013;1:27-42.
41. Paris V, Belloni A. Value in pharmaceutical pricing. OECD Health Working Papers, No. 63: OECD Publishing; 2013. doi.org/10.1787/5k43 jc9v6knx-en
42. Cassel D, Ulrich V. Ex-factory prices in European pharmaceutical markets as reimbursement framework of the German Statutory Health Insurance – issues and problems of international reference pricing for innovative pharmaceuticals. 22 February 2011. [cited 2015 Apr 24]. Available from: http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CDgQFjAA&url=http%3A%2F%2Fwww.vfa.de%2Fdownload%2Fexsum-cassel-ulrich.pdf&ei=NjwYVLm4\NdPmaKKKgsAJ&usg=AFQjCNG0icytL-w7KXQ_mWsYtHsWbDWVbw
43. European Commission. Ferrario A, Kanavos P. Managed entry agreements for pharmaceuticals: the European experience: LSE; April 2013 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://ec.europa.eu/enterprise/sectors/healthcare/files/docs/mea_report_en.pdf
44. Vogler S, Zimmermann N, Habl C, Piessnegger J, Bucsics A. Discounts and rebates granted to public payers for medicines in European countries. South Med Review. 2012;5(1):38-46.
45. Böttiger Y, Laine K, Andersson ML, Korhonen T, Molin B, Ovesjö ML, et al. SFINX-a drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol. 2009;65(6):627-33.
46. Sjoborg B, Backstrom T, Arvidsson LB, Andersen-Karlsson E, Blomberg LB, Eiermann B, et al. Design and implementation of a point-of-care computerized system for drug therapy in Stockholm metropolitan health region—Bridging the gap between knowledge and practice. Int J Med Inform. 2007;76(7):497-506.
47. Stausberg J, Hasford J. Drug-related admissions and hospital-acquired adverse drug events in Germany: a longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv Res. 2011;11:134.
48. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15-9.
49. Brvar M, Fokter N, Bunc M, Mozina M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol. 2009;9:8.
50. Rottenkolber D, Schmiedl S, Rottenkolber M, Farker K, Salje K, Mueller S, et al. Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf. 2011;20(6):626-34.
51. Miller I, Ashton-Chess J, Fert V, et al. Market access challenges in the EU for high medical value diagnostic tests. Personalised Medicine. 2011;8(2):137-48.
52. Vogler S, Zimmermann N, Leopold C, de Joncheere K. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Rev. 2011;4(2):69-79.
53. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76-87.
54. Godman B, Alvarez-Madrazo S, Acurcio F, Guerra Junior AA, Faridah Aryani MY, et al. Initiatives among authorities to improve the quality and efficiency of prescribing and the implications. J Pharma Care Health Sys. 2014;1(3):1-15.
55. Jelicic Kadic A, Zanic M, Skaricic N, Marusic A. Using the WHO essential medicines list to assess the appropriateness of insurance coverage decisions: a case study of the Croatian national medicine reimbursement list. PloS One. 2014;9(10):e111474.
56. Gustafsson LL, Wettermark B, Godman B, Andersen-Karlsson E, Bergman U, Hasselstrom J, et al. The ‘wise list’– a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108(4):224-33.
57. Björkhem-Bergman L, Andersén-Karlsson E, Laing R, Diogene E, Melien O, Jirlow M, et al. Interface management of pharmacotherapy. Joint hospital and primary care drug recommendations. Eur J Clin Pharmacol. 2013;69 Suppl 1:73-8.
58. Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9(1):65-83.
59. Serbian Health Insurance Fund. Wise List translated into Serbian. 2014 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://rfzo.rs/download/farmakopolitika/Mudra%20lista%201.%20deo.pdf
60. Hillner BE, Smith TJ. Efficacy does not necessarily translate to cost effectiveness: a case study in the challenges associated with 21st-century cancer drug pricing. J Clin Oncol. 2009;27(13):2111-3.
61. Evaluate Pharma. Surveying tomorrow’s BioPharma landscape. The NASDAQ Biotech index up close. June 2012 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://info.evaluatepharma.com/rs/evaluatepharmaltd/images/EvaluatePharma_NBI_Up_Close_2012.pdf
62. EFPIA. Health & Growth – Working together for a healthy Europe. A vision towards a life sciences strategy for Europe. 2014 [homepage on the Internet]. [cited 2015 Apr 24]. Available from: http://www.efpia.eu/uploads/documents/EFPIA-health&growth_MANIFESTO_V11_pbp.pdf
|
Author for correspondence: Brian Godman, BSc, PhD; Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, SE-14186, Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/pharmaceutical-pricing-in-croatia-a-comparison-of-ordinances-in-2013-versus-2009-and-their-potential-savings-to-provide-future-guidance.html
|
Abstract: |
Submitted: 28 November 2014; Revised: 3 December 2014; Accepted: 3 December 2014; Published online first: 16 December 2014
Vogler and co-authors have provided valuable insight into the procurement of generic medicines in hospitals among medium-sized European countries [1]. To date, this has been a neglected area with the majority of research and payer focus on ambulatory care because medicine expenditure in hospitals has only been a limited proportion of overall hospital expenditure at 5% to 10% over the years [1]. Pharmaceutical expenditure in ambulatory care is typically the second highest cost component after physician salaries, with expenditure rising by more than 50% in real terms during the past decade [2]. In addition, there are continual pressures on ambulatory care expenditure driven by well-known factors including ageing populations, rising patient expectations and the continued launch of new premium-priced technologies [2–4]. This has resulted in multiple policies and initiatives among authorities across Europe to enhance prescribing efficiency for both new and established medicines [2, 4]. Policies and initiatives for established medicines include encouraging the prescribing of lower cost generics versus originators and patented products in a class where all or nearly all the products in the class or related classes are seen as therapeutically similar [1, 2, 5, 6]. Classes include the proton pump inhibitors, renin-angiotensin inhibitor drugs as well as the statins [2, 5–7]. This takes advantage of an increasing number of products losing their patents in recent years [1, 2, 5, 6].
However, as Vogler and co-authors point out, the focus is changing with new premium-priced medicines, including biological medicines, initiated in hospitals before patients are discharged [1, 8]. This is a concern for the authorities responsible for ambulatory care if physicians are reluctant to change prescriptions to suitable lower cost generics, including generics versus originators, even in countries such as Germany where there is a high rate of generics prescribed [1, 8–10]. This is less of an issue if there is a tradition of International Nonproprietary Name (INN) prescribing across all sectors (UK) for small molecules [1, 6, 11], compulsory generics substitution (Sweden) [1, 6, 12], preference policies for the molecule (The Netherlands) [1, 2, 13], or reference pricing where patients have to cover the additional cost themselves for a more expensive medicine than the quality assured referenced priced medicine [1, 14]. However, this is an issue if patients are discharged on premium priced medicines, including originators, where low cost generics (or branded generics) are equally suitable and there is limited potential for switching in ambulatory care, enhanced by hospitals receiving appreciable discounts and sometimes free medicines [1, 15].
It was with this background, that the current study was conducted. It was encouraging to see a range of countries were studied in this paper with different healthcare systems, different geographies, different approaches to the tendering of medicines in hospital as well as different approaches to the pricing of generics in ambulatory care and measures to enhance their utilization [1]. In addition, concentrating on just one category of medicines, namely cardiovascular medicines, to provide good insights for the reasons stated. The study also built on the considerable contacts of the co-authors through the Pharmaceutical Health Information System (PHIS) network [1].
The study highlighted a number of interesting findings. These included the fact that typically hospitals only carry one product line, mainly the generic, or at the most two, i.e. both a generic and the originator, for a given medicine (only a minority of hospitals in Norway and Slovakia). The only exception was atorvastatin, where apart from Norway the originator was principally supplied. This compares to the ambulatory care sector where there may be multiple presentations available for dispensing from different companies once the patent has been lost [1]. One hospital in Portugal only carried atorvastatin rather than both simvastatin and atorvastatin [1]. This may reflect previously limited demand-side measures in ambulatory care in Portugal preferentially encouraging the prescribing of generic simvastatin rather than patented atorvastatin when it first became available, thereby encouraging the manufacturer of atorvastatin to seek its preferential listing in the hospital formulary [1, 16]. Secondly, just single doses are often supplied in hospitals versus typically full packs in the community with varying tablet sizes and strengths. Thirdly, Norway was the only country in which the surveyed medicines were exclusively centrally tendered leading to appreciable discounts. This provides an example to other countries, backed up by campaigns supporting generics [1]. There was also tendering among hospitals in Portugal, with individual hospitals having the potential to negotiate lower prices if able to do so [1]. Discounts of 100% were seen among the majority of surveyed hospitals in Austria, although discounts and cost-free medicines did not apply to new on-patent medicines. Otherwise, there was limited headroom for appreciable discounts between the hospitals [1]. Dispensing of originators in Austria will increase costs, with Austria having neither INN prescribing, generics substitution nor reference pricing [1, 17]. However, the sickness funds in Austria are looking to address this through information and other campaigns in hospitals [1, 18].
In conclusion, Vogler’s study offers valuable insight into the procurement of generics among hospitals in Europe once products lose their patent. It is hoped this study will be repeated for other product classes and other countries to provide further insight given the extensive networks and experience of the co-authors. This is especially important with growing recognition of the need for both ambulatory care and hospital sectors to work more closely together to maximize the health gain of patients with available resources – ‘Interface Management’ [1, 8].
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
1. Vogler S, Zimmermann N, Mazag J. Availability and procurement conditions of originator and generic medicines in hospitals – an exploratory study in five medium-sized European countries. Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(4):168-79. doi:10.5639/gabij.2014.0304.040.
2. Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
3. Garattini S, Bertele V, Godman B, Haycox A, Wettermark B, Gustafsson LL; Piperska Group. Enhancing the rational use of new medicines across European health care systems. Eur J Clin Pharmacol. 2008;64(12):1137-8.
4. Malmström RE, Godman BB, Diogene E, Baumgärtel C, Bennie M, Bishop I, et al. Dabigatran – a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;4:39.
5. Godman B, Wettermark B, Bishop I, Burkhardt T, Fürst J, Garuoliene K, et al. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
6. Godman B, Abuelkhair M, Vitry A, Abdu S, Bennie M, Bishop I, et al. Payers endorse generics to enhance prescribing efficiency; impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2014.0304.017.
7. Moon J, Godman B, Petzold M, Alvarez-Madrazo S, Bennett K, Bishop I, et al. Different initiatives across Europe to enhance losartan utilization post generics: impact and implications. Front Pharmacol. 2014;5:219. doi:10.3389/fphar.2014.00219. eCollection 2014.
8. Björkhem-Bergman L, Andersén-Karlsson E, Laing R, Diogene E, Melien O, Jirlow M, et al. Interface management of pharmacotherapy. Joint hospital and primary care drug recommendations. Eur J Clin Pharmacol. 2013;69 Suppl 1:73-8.
9. Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11(1):121-9.
10. Simmenroth-Nayda A, Hummers-Pradier E, Ledig T, Jansen R, Niebling W, Bjerre LM, et al. Prescription of generic drugs in general practice. Results of a survey of general practitioners [Article in German]. Med Klin (Munich). 2006;101(9):705-10.
11. Godman B, Bishop I, Finlayson AE, Campbell S, Kwon HY, Bennie M. Reforms and initiatives in Scotland in recent years to encourage the prescribing of generic drugs, their influence and implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):469-82.
12. Andersson KA, Petzold MG, Allebeck P, Carlsten A. Influence of mandatory generic substitution on pharmaceutical sales patterns: a national study over five years. BMC Health Serv Res. 2008;8:50.
13. Woerkom Mv, Piepenbrink H, Godman B, Metz Jd, Campbell S, Bennie M, et al. Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. J Comp Eff Res. 2012;1(6):527-38.
14. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries an overview. Generics and Biosimilars Initiative (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020.
15. Vogler S, Zimmermann N, Habl C, Mazag J. The role of discounts and loss leaders in medicine procurement in Austrian hospitals – a primary survey of official and actual medicine prices. Cost Eff Resour Alloc. 2013;11(1):15.
16. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):707-22.
17. Godman B, Burkhardt T, Bucsics A, Wettermark B, Wieninger P. Impact of recent reforms in Austria on utilization and expenditure of PPIs and lipid-lowering drugs: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2009;9(5):475-84.
18. Vogler S, Zimmermann N. How do regional sickness funds encourage more rational use of medicines, including the increase of generic uptake? A case study from Austria. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(2):65-75. doi:10.5639/gabij.2013.0202.027.
|
Author: Brian Godman, BSc, PhD, Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186, Stockholm, Sweden; Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/availability-and-procurement-of-generics-in-hospitals-among-medium-sized-european-countries.html
Author byline as per print journal: Brian Godman1,2,3,4, BSc, PhD; Andrew Hill5; Professor Steven Simoens6, MSc, PhD; Amanj Kurdi1,7, BSc, PhD; Jolanta Gulbinovič8, MD, PhD; Antony P Martin2,9; Angela Timoney1,10; Dzintars Gotham11, MBBS; Janet Wale12; Tomasz Bochenek13, MD, PhD; Celia C Rothe13; Iris Hoxha14; Admir Malaj15; Christian Hierländer16; Robert Sauermann16, MD; Wouter Hamelinck17; Zornitza Mitkova18; Guenka Petrova18, MPharm, MEcon, PhD, DSci; Ott Laius19; Catherine Sermet20; Irene Langer21; Gisbert Selke21; John Yfantopoulos22; Roberta Joppi23; Arianit Jakupi24; Elita Poplavska25, PhD; Ieva Greiciute-Kuprijanov26; Patricia Vella Bonanno1, BPharm, MSc, PhD; JF (Hans) Piepenbrink27; Vincent de Valk27; Carolin Hagen28; Anne Marthe Ringerud28; Robert Plisko29; Magdalene Wladysiuk29; Vanda Marković-Peković30,31, PhD; Nataša Grubiša32; Tatjana Ponorac33; Ileana Mardare34; Tanja Novakovic35; Mark Parker35; Jurij Fürst36; Dominik Tomek37, PharmD, MSc, PhD; Mercè Obach Cortadellas38; Corinne Zara38; Maria Juhasz-Haverinen39, MScPharm; Peter Skiold40; Stuart McTaggart41; Alan Haycox1, PhD
|
Introduction: There are appreciable concerns among European health authorities with growing expenditure on cancer medicines and issues of sustainability. The enhanced use of low-cost generics could help. |
Submitted: 5 February 2019; Revised: 14 March 2019; Accepted: 20 March 2019; Published online first: 2 April 2019
Despite the limited health gain for most new cancer medicines, their prices have increased appreciably in recent years [1–7]. This is reflected in the price per life year gained for new cancer medicines rising fourfold during the past twenty years after adjusting for inflation [2, 8]. Typically, prices for new cancer medicines are now approximately 6,000 to 9,000 Euros/patient/month and growing [1, 5, 9], with typically higher prices for new cancer medicines in the US versus Europe. As a result of rising prices for new cancer medicines, as well as increased prices for patented cancer medicines [5, 10–12], coupled with the increasing prevalence of cancer [1, 13], spending on cancer medicines in Europe has more than doubled in recent years, rising from Euros 8 billion in 2005 to Euros 19.1 billion in 2014 (at current prices) [14].
As a result, medicines for oncology now dominate pharmaceutical expenditures in developed markets, with projected worldwide sales for oncology medicines in 2017 ranging from US$74 to US$84 billion [15]. Overall, total worldwide sales of oncology medicines are expected to reach US$112 billion (Euros 91 billion) per year by 2020 [16]. Further growth is expected after this with cases of cancer worldwide likely to rise to 21.4 million per year by 2030 [1, 13] coupled more with than 500 companies actively pursuing new oncology medicines in over 600 indications, and looking to benefit from their investment [17]. The cost of cancer care also currently accounts for up to 30% of total hospital expenditure across diseases among European countries, and this is also rising with the launch of new premium-priced cancer medicines [14, 18].
Increasing prices for new cancer medicines, combined with increasing prevalence rates, is putting considerable strain on patients to access cancer treatments and healthcare systems to fund them [19–23]. High reimbursed prices for new cancer medicines certainly in Europe have been enhanced by the emotive nature of cancer, capturing public, physician, and political attention [13, 24–27]. If these trends with new cancer medicines continue, there is a real risk that universal healthcare across Europe will become unsustainable, which will not be in the best interest of any key stakeholder group. Initiatives among European health authorities to help fund future cancer treatments, including new valued high-cost medicines, include encouraging greater use of generic cancer medicines when they become available [12, 26]. This is especially important given the increasing number of cancer medicines that are now out of patent [28]. Initiatives to enhance greater use of generic cancer medicines include encouraging International Nonproprietary Name (INN) prescribing, financial incentives to enhance the prescribing and dispensing of generics versus originators, as well as compulsory generics substitution [29–38]. INN prescribing is supported by multiple publications showing no difference in effectiveness and safety between generics and originators across a range of disease areas including oncology medicines [28, 39–44]. There have though been concerns with generic imatinib due to different polymorphic forms between the originator and generics as early small scale studies suggested differences [45]. However, later studies involving more patients showed no difference in outcomes between generics and originators of imatinib [45, 46], and, typically, no substantial evidence that generic imatinib is less effective than the originator [47].
In general, prices for generic medicines in Europe are 20% to 80% below originator prices; however, some generics can be priced as low as 2% to 4% of the originator price before the patent was lost [48, 49]. As a result, substantial differences can occur in the prices of generics across Europe [37, 50, 51]. Overall, low prices can potentially be achieved for generic cancer medicines because of the low cost of goods that have been reported at just 1% or more of originator prices for some new cancer medicines [52, 53]. These low cost of goods have already resulted in considerable discounts for generic imatinib across countries versus pre-patent loss prices [54, 55]. However, this is not universal. For instance, in China, generic imatinib is only 10%–20% below originator prices although generic capecitabine is 50% lower than originator prices [56]. There have also been low prices for generic versions of paclitaxel in Europe at just over 1% of originator prices [57]. Docetaxel also has a low price in some European markets enhanced by an appreciable number of generic versions available [26]. Having said this, changes in the manufacturer have resulted in the prices of some low volume old anticancer medicines rising appreciably among European countries including Italy and the UK [58]. However, pharmaceutical companies are now being fined for such behaviour, e.g. Euros 5.2 million for Aspen in Italy for price increases for some of its anticancer medicines, with ongoing investigations and initiatives across Europe to address this [59, 60].
In some European countries, there have also been issues regarding the prescribing of certain generic medicines where there are different indications, one of which is still patent protected. This was the case with pregabalin for the treatment of neuropathic pain as opposed to epilepsy [61].
There have also been concerns with drug shortages if the prices of medicines including generics become too low, which is already happening for certain parenteral medicines [62]. However, these concerns have to be balanced against the increasing availability and use of low-cost generic medicines to release resources to fund increased cancer care including new innovative medicines, reducing patient co-payments where these exist, and stimulating innovation [13, 48, 63–65].
Consequently, there is a need to document current regulations surrounding the pricing of generic oral anticancer medicines across Europe, and their impact on subsequent prices, as well as issues regarding prices of medicines once one indication loses its patent but not the others. As a result, the aims of this paper are multiple. The primary aim is to document current arrangements for the pricing of oral generic medicines across Europe and whether there are any differences in pricing policies for generic cancer medicines versus those for other disease areas. This is important to maximize savings following generics availability as we do see differences in reimbursement decisions for new medicines for cancer versus those for other disease areas [27, 66]. Secondly, to investigate what happens to the prices of oral cancer medicines if one indication loses its patent but not the other indication(s) since indication-specific pricing will undoubtedly reduce potential savings following generics availability, and, as mentioned, there were issues with the prescribing of pregabalin across indications when the first indication lost its patent [61]. Thirdly, to investigate how payers and their advisers envisage developments in the pricing of oral generic medicines for cancer as more cancer medicines lose their patents. Fourthly, to assess whether health authorities have any concerns with patient safety when prescribing oral generic cancer medicines similar to the situation with medicines with a narrow therapeutic index, such as lithium and certain medicines for epilepsy [67–69]. Fifthly, to review current prices for a number of oral cancer medicines across Europe where generic versions are available in all or some of the countries. This will also include an evaluation of prices over time to fully assess the impact of the different pricing arrangements for generic cancer medicines across Europe since we are aware that prices of generics can vary substantially across Europe due to different policies [26, 37, 48, 49], and that Central and Eastern European (CEE) countries are likely to have generics earlier [70]. Sixthly, to assess whether there are any differences in the prices of generics by population size and geography (CEE versus Western European countries) as there have been concerns that countries with small populations, and hence lower economies of scale, may have difficulty negotiating low prices for medicines [71] ; however, published literature has not found to be the case [31, 72]. Countries with greater economic power may potentially be able to negotiate lower prices for generics, and we will also investigate this. Lastly, as mentioned, prices of some old low volume anticancer medicines have risen among European countries in recent years with changes in the manufacturer [58], and we will assess the impact of any changes in manufacturers on subsequent prices, and the likely future direction.
We developed a number of hypotheses for the different aims. These include:
We believe that this is the first time that such comprehensive research regarding the regulations surrounding the pricing of oral cancer medicines, and their influence on subsequent prices, has been undertaken across Europe. Consequently, we believe the findings will inform future discussions within and between European and other countries regarding the pricing of oral generic cancer medicines now and into the future. In addition, we aim to stimulate discussions regarding subsequent prices and/or discounts for still patented oral cancer medicines that used off-patented products as their comparator during pricing negotiations. As a result, help with issues of affordability and sustainability of medicine use in the future in this high priority disease area.
When we refer to ‘generics’, we mean the chemical entity (INN). This study also includes imatinib, a comparatively newer anticancer agent, which is treated similarly to other oral generic cancer medicines. Biosimilars of originator biological medicines are regulated by a different framework, and incentives to increase their use are different [73]. Consequently, we will not be discussing biosimilars.
We have chosen to concentrate on Europe in view of the high use of generic medicines in this region. There are also different approaches to the pricing of generics among the various countries, as well as an appreciable number of existing initiatives to enhance the prescribing of generic medicines.
A mixed method approach was employed including both qualitative and quantitative research to meet the study objectives, and included 25 European countries, see Box 1.
This appreciable number of European countries fully encompasses differences in geography, population size, gross domestic product (GDP) per capita, pricing approaches towards generics as well as different approaches to the financing of healthcare [30, 70, 74]. Countries were also broken down into CEE and Western European countries based on the Organisation for Economic Co-operation and Development (OECD) definition [75] to ascertain any differences in the pricing of oral generic cancer medicines based on population sizes and market power.
Qualitative interviews were undertaken with senior personnel within health authorities including heads of pricing and reimbursement of medicines as well as their advisers and a limited number of academics with expertise on national pharmaceutical issues and other key issues among European countries. The involvement of these senior level personnel in the qualitative interviews, and their involvement as co-authors, is seen as very important to enhance the accuracy, robustness and insight of the replies as there have been concerns when such approaches are not used [76, 77]. The co-authors were either identified via the Piperska group [78, 79] – a multidisciplinary network of professionals with interest in the quality use of medicines – or had been previous co-authors on similar pan-European projects involving generic medicinal products [29, 80–82]. We have successfully used this approach in other key areas [30, 61, 70, 73, 74, 80, 81, 83].
The qualitative questions to address the identified objectives included:
If applicable, pricing policies for generics were broken down into three principal groups to aid comparisons. This builds on previous publications that have used this approach [84–87], and include:
Health authority and health insurance company databases were used for the quantitative research apart from Greece and Serbia where commercial sources were used. The information within these databases is robust as they are typically used to trigger payment to pharmacists. In addition, the databases are regularly audited, and cover the whole country rather than a sample of pharmacies as seen with commercial databases. We have used this approach before when conducting research on prices and utilization of generics, originators and patented products within a class or related class [30, 49, 70, 72, 80–82, 88].
The oral cancer medicines selected for the quantitative research were busulfan (L01AB01), capecitabine (L01BC06), chlorambucil (L01AA02), cyclophosphamide (L01AA01), flutamide (L02BB01), imatinib (L01XE01), melphalan (L01AA03), methotrexate (L01BA01), and temozolomide (L01AX03) [89]. These oral cancer medicines were chosen to provide a range of medicines for use across different cancer types, with typically multiple indications, covering the oral cancer medicines listed in the World Health Organization (WHO) essential medicines list, and exhibiting varying timings regarding the loss of patent, building on previous publications [55]. Oral medicines, such as gefitinib, sorafenib, sunitinib and tioguanine were not included as there appeared to be no generics yet available for these oral cancer medicines even among the selected CEE countries at the time of the study. The (non-) tyrosine kinase inhibitors are a particular case as there are typically multiple indications as well as potentially different dates for patent loss across countries [90].
Since the perspective of this study is that of health authorities, reimbursed prices were principally chosen for comparative purposes rather than total prices, which include patient co-payments. Reimbursed prices can include prices after deducting legally mandated discounts; however, this is unlikely for generic medicines. In a minority of countries, e.g. Kosovo, procured and total prices were used as reimbursed prices were unavailable. In Italy and Norway, reimbursed prices were also listed but the medicines are typically dispensed in hospitals where further confidential discounts are provided. However, since these discounts are confidential, we could only report ambulatory care prices. Furthermore, in some countries, prices did not include VAT and/or pharmacy margins, e.g. Malta, The Netherlands and the Republic of Srpska. However, this was not seen to have an appreciable impact on the analyses since percentage reductions were typically used for comparative purposes to assess the influence of different policies. In addition, the prices of generics at least initially in a number of European countries are based on percentage reductions from pre-patent loss originator prices [30, 70]. We have successfully used this approach in a number of previous publications [30, 70, 74, 80, 84].
We are conscious that we used prices from Scotland rather than the UK as a whole. However, there are no differences in prices across the UK with reimbursed prices in ambulatory care based on the tariff price coupled with free-market competition, which leads to further price reductions over time. Similarly, no differences are envisaged in generic and originator drug prices in ambulatory care in Catalonia versus Spain as a whole. However, we acknowledge that there will be differences in hospital prices across Spain through variations in discounts and managed entry agreements between the different regions [91].
Prices were collected over a period of time between 2013 and 2017, based on the unit, e.g. tablet strength, for the different originators and the cheapest generics substitute unit available, especially for branded generics, as opposed to defined daily doses (DDDs). Unit strength was chosen as generally there are no DDDs for oral cancer medicines in view of typically a number of indications for each molecule [89]. The documented prices for each year between 2013 and 2017 were the last available price for that year, e.g. October to December if prices are adjusted every three months or December if prices are adjusted monthly. The years were chosen to assess the extent of any price changes in recent years especially given recent price increases for some off-patent medicines.
The actual unit strength chosen for comparative purposes reflects the most used strength in a number of European countries, although we recognize that there may be different strengths available in some countries. In addition:
Initially, prices were documented in the country’s currency if this was not Euros. Subsequently where relevant, prices were converted to Euros for the purposes of comparison based on current exchange rates and validated with the co-authors to enhance the robustness of the findings, see Table 1A in the Appendix.
Table 2A in the Appendix contains details of the population sizes and the breakdown of countries into three groups for analysis purposes (small, medium and large). Countries with populations under three million were described as small, those with populations between three and 16 million as medium, and the remainder as large to produce three roughly equal groups although recognizing that more countries were in the ‘small’ category than in the others.
Statistical tests were performed to ascertain any trends in prices across countries as well as any difference in price reductions over time as a result of the different approaches towards the pricing of generics across Europe. The significance threshold was set at p > 0.05. Statistical tests were also run to check whether prices were influenced by population size as this has been a concern in the past. However, we did not have enough variables to perform any multivariate analyses as there are not enough specific measures in each European country that had been initiated to control the price of generic cancer medicines which could be used as a yes/no variable. We have though collated the impact of the different pricing approaches for generic medicines across Europe as one of the principal objectives of this paper was to document these and their influence on subsequent prices for oral generic cancer medicines.
No analysis was made regarding the impact of volumes on generic drug prices as seen in other studies because this was not a focus of the paper [92]. This will be followed up in future studies. Potential savings from the increased utilization of generic medicines for cancer over time will also form a separate project building on previous calculations [93].
In line with the objectives of this paper, we will first report the findings from the qualitative research. This will be followed by the findings from the quantitative research.
Regulations surrounding the pricing of generics including those for cancer across Europe
Regulations for the pricing of generics generally and for oral cancer medicines including likely changes in the short term
As previously seen, there were differences in the approaches to the pricing of generics among the various European countries, see Table 1, which could again be categorized under three broad headings. These are: (i) prescriptive pricing approaches; (ii) market forces, and (iii) and a mixture of the approaches.
As shown in Table 1, there were no differences in the pricing approaches for generic oral medicines for oncology compared with those for other disease areas, see Table 1, apart from the fact that in some countries these cancer medicines were dispensed in hospitals, which could include additional discounts that are typically confidential. This is important given the considerable savings that can be achieved with the introduction of generics, see Table 2.
Pricing of oral cancer medicines if one indication has lost its patent
There were generally no issues with the pricing and usage of oral generic cancer medicines once one indication had lost its patent but not the other indication(s), see Table 1; however, this was not universal. There is similar to the situation in Germany once the first indication for a multiple-sourced product has lost its patent [61], i.e. the generic medicine can be prescribed across all indications at the discounted price versus the originator. However, this is different to the situation that existed with pregabalin among a number of European countries where the generics could only be prescribed for epilepsy and the originator must be prescribed and reimbursed accordingly for neuropathic pain [61].
This situation is encouraging for maximiẕing savings following generic drug availability given the considerable savings that can be achieved with the introduction of generics, see Table 2, and will be closely monitored in the future. This includes procured medicines for distribution in hospitals.
Substitution of oral cancer medicines and initiatives to encourage their prescribing
It was also encouraging to see that once generics became available, there were no concerns with substitution with the selected oral generic cancer medicines, see Table 1. This is similar to the general situation for cancer medicines [28]. Consequently, originators can be substituted with generics without compromising care. This is again important for maximizing savings once generics become available.
Initiatives to encourage the prescribing of generics in preference to originator cancer medicines among the various European countries, see Box 2, were similar to those for generic medicines in general.
Price reductions with generics over time and with originators, as well as the influence of population size and market forces
Prices for originator 400 mg imatinib were similar in Western European countries in 2015 before generic drug availability, see Figure 1. Generic imatinib was already available in CEE countries, e.g. in Albania, Estonia, Latvia, Lithuania, Romania, Serbia and Slovakia, on or before 2013, and in Poland and Slovenia in 2014. Generic capecitabine and flutamide were already available in a number of Western European countries in 2013. The other three oral anticancer medicines were already available as generics among Western European countries before 2013.
As expected, there were differences in the prices of the generics for capecitabine (500 mg), flutamide (250 mg), imatinib (400 mg) and temozolomide (20 mg and 250 mg) in 2017 among the European countries where these generics were available, see Table 3, reflecting differences in the approaches to the pricing of oral generics among the various European countries, see Table 1.
There were no differences in the prices of capecitabine (500 mg), flutamide (250 mg), imatinib (400 mg) and temozolomide (20 mg and 250 mg) when the countries were broken down into small, medium or large populations, see Table 2A, apart from generic imatinib for European countries with large populations, i.e. capecitabine (p = 0.372 for medium and p = 0.100 for large), flutamide (p = 0.700 for medium and 0.385 for large), imatinib (p = 0.249 for medium and p = 0.037 for large), temozolomide 20 mg (p = 0.764 for medium and p = 0.085 for large) and temozolomide 250 mg (p = 0.951 for medium and p = 0.105 for large). Prices for generic capecitabine (p = 0.007), generic imatinib (p = 0.015) and temozolomide 20 mg (p = 0.015) were though typically appreciably higher when combined among Western European countries compared with CEE countries. However, this was not the case for flutamide 250 mg or temozolomide 250 mg. This will change for generic imatinib as prices fall among the large Western European countries over time, e.g. generic imatinib in Scotland by March 2018 was already 89.1% below pre-patent loss prices having only been made available in 2016.
Overall in 2017, the differences in the prices of generics of capecitabine (500 mg), flutamide (250 mg), imatinib (400 mg) and temozolomide (20 mg and 250 mg) among the different European countries, see Table 3, appear to be a reflection of the differences in the country’s pricing approaches towards generics, see Table 1, rather than population size when broken down into small, medium or large, as well as the timing of generic drug availability. These findings are similar to those with generic busulfan, chlorambucil, cyclophosphamide and melphalan, see Table 4, and confirm this trend certainly for oral generic cancer medicines.
There also appears to be no correlation in terms of the price reductions seen for generic capecitabine, flutamide, imatinib or temozolomide in 2017 versus 2013 originator prices with population sizes, i.e. appreciable price reductions over time were not confined to European countries with higher populations, see Table 2. There was also no significant difference in the percentage reduction between Western and CEE countries for capecitabine, flutamide, imatinib or temozolomide.
If anything, there was a greater reduction in the prices of generic capecitabine, see Figure 2, in countries with a mixed approach to the pricing of generics versus those with market forces (p = 0.035) and prescriptive (p = 0.041) approaches.
This was not the case for generic imatinib, see Figure 3, although there was a trend (p = 0.079 for market forces and p = 0.119 for prescriptive pricing approaches). However, this may change as prices of generic imatinib fall in The Netherlands, Spain, Sweden and the UK, e.g. as mentioned generic imatinib in Scotland was already 89.1% below pre-patent loss prices by March 2018 having only been made available in 2016.
Price changes for generics with changes in the licence holder
In recent years, the generic drug manufacturer Aspen has taken over the licence of busulfan, chlorambucil, and melphalan and enjoyed market monopoly, with typically no generics competition, despite the loss of patent protection. Another pharmaceutical company, Baxter, has used a similar strategy with cyclophosphamide. Countries have typically been faced with having to either accept these higher prices or no longer procuring and/or reimbursing these medicines.
Figure 4 depicts current prices per tablet for these four oral generic anticancer medicines (2017) where these are currently listed and reimbursed since not all European countries currently reimburse these four medicines. There were no significant differences in the mean prices for these four anticancer medicines between Western and CEE countries. In the case of busulfan, there was a difference in current prices among European countries, with Western European countries typically having higher prices but this did not reach significance (p = 0.08). Population size again did not appear to influence subsequent prices.
There were appreciable price rises in a number of other European countries following Aspen’s purchase of busulfan, chlorambucil and melphalan from GlaxoSmithKline, see Table 4.
Similar price rises were seen in Germany during this period, for example:
Overall there again appears to be no link between prices, geographic region and population size for these four medicines despite previous concerns. If anything, countries with smaller populations, such as Estonia had lower prices than Belgium, Italy and the UK. This appears to be in contrast with previous publications [71], although this is in accordance with previous findings in Lithuania and the Republic of Srpska [31, 72].
The difference in prices of these four molecules between the countries in 2017 may well reflect differences in negotiations when the new company took over the molecules and relaunched them as a new originator at a higher price. Countries such as Estonia appeared successful in negotiating lower price increases for the relaunched medicines versus for instance Belgium, Italy and the UK. However, as mentioned earlier, the company was subsequently fined Euros 5.2 million in Italy for their activities [59]. Currently, Germany has a moratorium law stating that for any price increase, there will be an increase in the mandatory rebate of exactly the same amount as the increase. Effectively, this means that the price paid by statutory heathcare funds for ambulatory care medicines does not change [114, 115]. Legislation is also being introduced in the UK to reduce the price of generic medicines where competition fails to reduce prices and companies are seen to charge the National Health Service (NHS) unreasonably high prices [113]. The Competition and Markets Authority (CMA) in the UK has also provisionally found that two companies broke the law by agreeing not to compete to supply generic hydrocortisone to the NHS driving up annual costs by two million GBP [116]. There are also ongoing deliberations in Europe regarding concerns with appreciable price increases for generics and whether this breaks EU competition rules [60, 117]. In addition, we may see countries combining to form regional co-operations between Member States to further reduce prices where there are concerns with unjustified price rises building on current consortia [118–120]. However, this has to be balanced against issues of future profitability and potential drug shortages with for instance only 75 melphalan tablets dispensed in ambulatory care in Scotland during the study period (SM personal communication), and with just 11 boxes of generic melphalan dispensed in Serbia in 2015.
We believe this is the first study to comprehensively research the situation regarding the pricing of oral generic cancer medicines in this high priority disease area. We believe our findings again highlight the differences that are seen in the various approaches to the pricing of generics across Europe, see Table 1, and their subsequent influence on the prices of generics and discounts obtained. These considerable differences are in direct contrast with the more limited differences in prices for on-patent medicines across Europe, especially high cost ones [121], although these can still be considerable [122].
Prices for generic capecitabine, imatinib, and temozolomide 20 mg, were significantly higher when combined among Western European countries as compared with CEE countries. This may reflect greater availability of resources to spend on medicines among Western countries. However, this is not universal as there were no differences in prices between Western European and CEE countries for flutamide 250 mg or temozolomide 250 mg. Alternatively, generics may be available earlier in CEE countries with associated earlier falls in their price as seen with imatinib. As mentioned, generic imatinib in Scotland was already 89.1% below pre-patent loss prices in March 2018 having only been made available in 2016, see Figure 1. Having said this, there were already some appreciable price reductions for these various molecules and strengths among Western European countries comparing generic drug prices in 2017 versus originator prices in 2013, Table 3 and Figure 3. In addition, no overall difference in the price reductions for these molecules and strengths was found for Western European countries combined versus CEE countries combined.
The picture regarding methotrexate is complicated by the use of this medicine for immunological conditions, such as rheumatoid arthritis and psoriasis. This meant that different originator and tablet strengths (2.5 mg or 5 mg) were available amongst the European countries making comparisons difficult. In addition, the considerable time that both the originator and generic methotrexate have been available across Europe meant there were limited price reductions in reality between 2017 compared with 2013 among the various European countries. Consequently, no detailed analysis was undertaken with methotrexate.
The price reductions for capecitabine, flutamide, imatinib and temozolomide that have been achieved in practice, see Table 3, confirm the findings of Hill et al. that the costs of goods for cancer medicines can be very low in reality [52, 53]. This fuels the debate for greater transparency in the pricing of new medicines for cancer when pharmaceutical companies request premium prices especially where there is limited health gain for their new cancer medicines versus current standards.
In view of our findings, we also believe European health authorities, as well as health authorities from other countries, can use these results to reassess their pricing approaches for generics, and the subsequent implications for oral generic cancer medicines, given concerns with the increasing costs of medicines to treat patients with cancer and issues of sustainability [3, 9, 28]. This is already happening, and may well accelerate, especially if issues of access and sustainability of cancer care continue to be priority issues for all key stakeholder groups. For instance, Austria, Lithuania, and Sweden, have recently introduced measures to further lower the prices they pay for generics and this trend is likely to continue, see Table 1. However, this has to be balanced against issues of parallel exports and associated drug shortages if the prices of generics become too low [37, 123], which is depicted in the unavailability of some oral generic cancer medicines in the countries studied. This will be the subject of ongoing research.
Based on our findings combined with the continual pressure on oncology budgets, we would also likely to see countries increasingly reassess the prices of on-patent cancer medicines in their country once the comparator medicine used for pricing and reimbursement negotiations loses its patent [124]. Such activities will be enhanced by the low prices that have been achieved for oral cancer medicines once their patent is lost, see Tables 2 and 3, which will continue.
Encouragingly, we saw no differences in the pricing approaches for generic cancer medicines versus those for other disease areas despite the emotive nature of cancer, and this will continue. This is essential to maximize savings from the availability of generic cancer medicines once available, with ongoing initiatives across Europe to encourage their use where pertinent. Likely, current and future initiatives to enhance the prescribing of oral generic cancer medicines include additional demand-side measures especially among European countries with currently low use of generics versus originators. This could be alongside continued educational initiatives among key stakeholder groups, including patients, where pertinent about the lack of problems with oral generic cancer medicines given there were no concerns with substitution among the European countries surveyed, see Table 1.
We are also unlikely to see major changes in pricing approaches once one indication loses its patent. The situation seen in Germany, as well as initiatives to encourage INN prescribing, helps in this regard. These initiatives are essential in oncology given the growing burden of the cost of medicines to treat patients with cancer across Europe combined with the need to continue to provide universal healthcare. Encouraging greater prescribing of generic medicines will be helped by limited or no fears with substituting generic cancer medicines for originators among physicians and pharmacists. This is unlike a limited number of other disease areas including some medicines for patients with epilepsy as well as lithium for patients with certain mental health conditions [67–69].
If companies continue to purchase the patent for old cancer medicines and other products, and relaunch them at considerably higher prices, there is likely to be co-ordinated activities across Europe to try and address this. We are already seeing companies being fined, e.g. Italy, as well as countries instigating measures to reduce any burden from such approaches as seen currently in Germany and the UK. Such punitive actions are likely to grow in the future if this trend continues. However, this has to be balanced against potential availability especially if only small volumes are being used. This will again be the subject of continuing research.
We have again seen appreciable differences in the regulations surrounding the pricing of generics across Europe, reflected in different reimbursed prices for oral generic cancer medicines. Welcomed from an equity and resource perspective were no differences in the pricing approaches for medicines for cancer as opposed to other disease areas to help maximize savings once generics become available. In addition, the prices of generics, and any difference in the prices of generics in 2017, or price reductions versus 2013 originator prices, did not appear to be influenced by population size. This is also encouraging to maximize savings from the availability of oral generic cancer medicines. The prices of some generics were higher among Western European countries in 2017; however, this could have been influenced by generics being available earlier among CEE countries. There are concerns with some off-patented medicines being relaunched by alternative companies at appreciably higher prices, although there are ongoing steps across Europe to try to address this. These initiatives are likely to grow if this trend continues.
Reassuringly, there are no concerns with substitution of oral generic medicines. In addition, prices were typically similar across indications. Both results are important to maximiẕe savings from generic drug availability once at least one indication loses its patent.
We have tried to reduce limitations with this study by using senior level personnel for the qualitative research as well as health authority and health insurance company databases for the quantitative research. However, we are aware that we did not use reimbursed prices throughout the countries, some prices did not include VAT, and we cannot be sure that the medicines chosen for the research are entirely used for cancer indications. Despite these concerns, we believe the findings of our comprehensive research are robust providing direction not only to key stakeholder groups across Europe but also wider.
There was no funding for this research and no assistance with the write-up.
Competing interests: Most of the authors work directly for health authorities or health insurance companies or are advisers to them. Professor Steven Simoens has previously held the EGA Chair ‘European policy towards generic medicines’. All the authors have no other conflicts of interest to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK
2Health Economics Centre, University of Liverpool Management School, Liverpool, UK
3Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, SE-141 86, Stockholm, Sweden
4School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang, Malaysia
5Institute of Translational Medicine, University of Liverpool, UK
6KU Leuven Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium
7Department of Pharmacology, College of Pharmacy, Hawler Medical University, Erbil, Iraq
8Department of Pathology, Forensic Medicine and Pharmacology, Institute of Biomedical Sciences, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
9HCD Economics, The Innovation Centre, Daresbury, WA4 4FS, UK
10NHS Lothian, Edinburgh, UK
11Independent researcher, Boston, MA, USA
12Independent consumer advocate, 11a Lydia Street, Brunswick, Victoria 3056, Australia
13Department of Drug Management, Faculty of Health Sciences, Jagiellonian University Medical College, Krakow, Poland
14Department of Pharmacy, Faculty of Medicine, University of Medicine, Tirana, Albania
15University of Medicine, Tirana, Albania
16Main Association of Austrian Social Security Institutions, Department of Pharmaceutical Affairs, 1 Haidingergasse, AT-1030 Vienna, Austria
17Statistics Department, APB, 11 Rue Archimède, BE-1000 Bruxelles, Belgium
18Faculty of Pharmacy, Department of Social Pharmacy and Pharmacoeconomics, Medical University of Sofia, Sofia, Bulgaria
19State Agency of Medicines, 1 Nooruse, EE-50411 Tartu, Estonia
20IRDES, 117 bis rue Manin, FR-75019 Paris, France
21Wissenschaftliches Institut der AOK (WidO), 31 Rosenthaler Straße, DE-10178 Berlin, Germany
22School of Economics and Political Science, University of Athens, Athens
23Pharmaceutical Drug Department, Azienda Sanitaria Locale of Verona, Verona, Italy
24UBT – Higher Education Institute Prishtina, Kosovo
25Institute of Public Health & Faculty of Pharmacy, Riga Stradins University, Latvia
26Department of Pharmacy, Ministry of Health of the Republic of Lithuania, Vilnius, Lithuania
27National Health Care Institute (ZIN), 4 Eekholt, NL-1112 XH Diemen, The Netherlands
28HTA and Reimbursement, Norwegian Medicines Agency, Oslo, Norway
29HTA Consulting, 17/3 Starowis′lna Str, PL-31-038 Cracow, Poland
30Ministry of Health and Social Welfare, Banja Luka, Republic of Srpska, Bosnia and Herzegovina
31University of Banja Luka, Faculty of Medicine, Department of Social Pharmacy, Banja Luka, Republic of Srpska, Bosnia and Herzegovina
32Health Insurance Fund of Republika Srpska, 8 Zdrave Korde, 78000 Banja Luka, Republic of Srpska, Bosnia and Herzegovina
33Agency for medicines and medical devices of Bosnia and Herzegovina, Veljka Mladjenovica bb, 78000 Banja Luka, Bosnia and Herzegovina
34Faculty of Medicine, Public Health and Management Department, ‘Carol Davila’ University of Medicine and Pharmacy Bucharest, RO-050463 Bucharest, Romania
35ZEM Solutions, 9 Mosorska, RS-11 000 Belgrade, Serbia
36Health Insurance Institute, 24 Miklosiceva, SI-1507 Ljubljana, Slovenia
37Faculty of Medicine, Slovak Medical University in Bratislava, Bratislava, Slovakia
38591 Gran Via de les Corts Catalanes, 4a place, ES-08007 Barcelona, Spain
39Stockholm County Council, Stockholm, Sweden
40TLV (Dental and Pharmaceutical Benefits Agency), 18 Fleminggatan, SE-10422, Stockholm, Sweden
41NHS National Services Scotland, Gyle Square, 1 South Gyle Crescent, Edinburgh EH12 9EB, UK
References
1. Kelly RJ, Smith TJ. Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. 2014;15(3):e112-8.
2. Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29(1):139-62.
3. Godman B, Wild C, Haycox A. Patent expiry and costs for anticancer medicines for clinical use. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(3):105-6. doi:10.5639/gabij.2017.0603.021
4. Prasad V, Wang R, Afifi SH, Mailankody S. The rising price of cancer drugs-a new old problem? JAMA Oncol. 2016. doi:10.1001/jamaoncol.2016.4275. [Epub ahead of print]
5. Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium–the just price. J Clin Oncol. 2013;31(28):3600-4.
6. Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
7. Grössmann N, Wild C. Between January 2009 and April 2016, 134 novel anticancer therapies were approved: what is the level of knowledge concerning the clinical benefit at the time of approval? ESMO Open. 2017;1(6):e000125. doi:10.1136/esmoopen-2016-000125.
8. Bach PB, Saltz LB. Raising the dose and raising the cost: the case of pembrolizumab in lung cancer. J Natl Cancer Inst. 2017;109(11).
9. Godman B, Oortwijn W, de Waure C, Mosca I, Puggina A, Specchia ML, et al. Links between pharmaceutical R&D models and access to affordable medicines. A study for the ENVI Committee.
10. Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000-2014. JAMA Oncol. 2016;2(7):960-1.
11. Gordon N, Stemmer SM, Greenberg D, Goldstein DA. Trajectories of injectable cancer drug costs after launch in the United States. Journal of clinical oncology. 2017:Jco2016722124.
12. Gyawali B, Sullivan R. Economics of cancer medicines: for whose benefit? New Bioeth. 2017;23(1):95-104.
13. Chalkidou K, Marquez P, Dhillon PK, Teerawattananon Y, Anothaisintawee T, Gadelha CA, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol. 2014;15(3):e119-31.
14. Wilking N, Lopes G, Meier K, Simoens S, van Harten W, Vulto AG. Can we continue to afford access to cancer treatment? Eur Oncol Haematol. 2017;13(2):114-9.
15. Lindsley CW. New 2016 data and statistics for global pharmaceutical products and projections through 2017. ACS Chem Neurosci. 2017;8(8):1635-6.
16. Allied Market Research. Oncology/cancer drugs market by therapeutic modalities (chemotherapy, targeted therapy, immunotherapy, hormonal), cancer types (blood, breast, gastrointestinal, prostate, skin, respiratory/lung cancer) – global opportunity analysis and industry forecast, 2013-2020 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.alliedmarketresearch.com/oncology-cancer-drugs-market?oncology-cancer-drugs-market
17. IMSH Institute for Healthcare Informatics. Global Oncology Trend Report. A review of 2015 and outlook to 2020. June 2016. Available from: https://www.scribd.com/document/323179495/IMSH-Institute-Global-Oncology-Trend-2015-2020-Report
18. Simoens S, van Harten W, Lopes G, Vulto A, Meier K, Wilking N. What Happens when the cost of cancer care becomes unsustainable. Eur Oncol Haematol. 2017;13(2):108-13.
19. Tefferi A, Kantarjian H, Rajkumar SV, Baker LH, Abkowitz JL, Adamson JW, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90(8):996-1000.
20. Sarwar MR, Iftikhar S, Saqib A. Availability of anticancer medicines in public and private sectors, and their affordability by low, middle and high-income class patients in Pakistan. BMC Cancer. 2018;18(1):14.
21. Atieno OM, Opanga S, Martin A, Kurdi A, Godman B. Pilot study assessing the direct medical cost of treating patients with cancer in Kenya; findings and implications for the future. J Med Econ. 2018;21(9):878-87.
22. Jakupi A, Godman B, Martin A, Haycox A, Baholli I. Utilization and expenditure of anti-cancer medicines in Kosovo: findings and implications. Pharmacoecon Open. 2018;2(4):423-32.
23. Ghinea H, Kerridge I, Lipworth W. If we don’t talk about value, cancer drugs will become terminal for health systems. The Conversation. 2015 Jul 26.
24. Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381-90.
25. Goldstein DA, Clark J, Tu Y, Zhang J, Fang F, Goldstein R, et al. A global comparison of the cost of patented cancer drugs in relation to global differences in wealth. Oncotarget. 2017;8(42):71548-55.
26. World Health Organization. Hoen E’t. Access to cancer treatment: a study of medicine pricing issues with recommendations for improving access to cancer medication [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://apps.who.int/medicinedocs/documents/s21758en/s21758en.pdf
27. Haycox A. Why cancer? Pharmacoecon. 2016;34(7):625-7.
28. Yang YT, Nagai S, Chen BK, Qureshi ZP, Lebby AA, Kessler S, et al. Generic oncology drugs: are they all safe? Lancet Oncol. 2016;17(11):e493-e501.
29. Godman B, Wettermark B, van Woerkom M, Fraeyman J, Alvarez-Madrazo S, Berg C, et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: findings and future implications. Front Pharmacol. 2014;5:106.
30. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):707-22.
31. Garuoliene K, Godman B, Gulbinovic J, Wettermark B, Haycox A. European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
32. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
33. Dylst P, Vulto A, Godman B, Simoens S. Generic medicines: solutions for a sustainable drug market? Appl Health Econ Health Pol. 2013;11(5):437-43.
34. Babar ZU, Kan SW, Scahill S. Interventions promoting the acceptance and uptake of generic medicines: a narrative review of the literature. Health Policy. 2014;117(3):285-96.
35. Moe-Byrne T, Chambers D, Harden M, McDaid C. Behaviour change interventions to promote prescribing of generic drugs: a rapid evidence synthesis and systematic review. BMJ Open. 2014;4(5):e004623.
36. Kaplan WA, Ritz LS, Vitello M, Wirtz VJ. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000-2010. Health Policy. 2012;106(3):211-24.
37. Wouters OJ, Kanavos P, McKEE M. Comparing generic drug markets in Europe and the United States: prices, volumes, and spending. Milbank Q. 2017;95(3):554-601.
38. Bucek Psenkova M, Visnansky M, Mackovicova S, Tomek D. Drug policy in Slovakia. Value Health Reg Issues. 2017;13:44-9.
39. Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300(21):2514-26.
40. Lessing C, Ashton T, Davis PB. The impact on health outcome measures of switching to generic medicines consequent to reference pricing: the case of olanzapine in New Zealand. J Prim Health Care. 2015;7(2):94-101.
41. Veronin M. Should we have concerns with generic versus brand antimicrobial drugs? A review of issues. JPHSR. 2011;2:135-50.
42. Paton C. Generic clozapine: outcomes after switching formulations. Br J Psychiatry. 2006;189:184-5.
43. Corrao G, Soranna D, Arfe A, Casula M, Tragni E, Merlino L, et al. Are generic and brand-name statins clinically equivalent? Evidence from a real data-base. Eur J Internal Med. 2014;25(8):745-50.
44. Corrao G, Soranna D, Merlino L, Mancia G. Similarity between generic and brand-name antihypertensive drugs for primary prevention of cardiovascular disease: evidence from a large population-based study. Eur J Clin Invest. 2014;44(10):933-9.
45. Mathews V. Generic imatinib: the real-deal or just a deal? Leuk Lymphoma. 2014;55(12):2678-80.
46. Malhotra H, Sharma P, Bhargava S, Rathore OS, Malhotra B, Kumar M. Correlation of plasma trough levels of imatinib with molecular response in patients with chronic myeloid leukemia. Leuk Lymphoma. 2014;55(11):2614-9.
47. Canadian Association of Pharmacy in Oncology. de Lemos M, Kletas V. Generic imatinib: review of the literature on clinical efficacy [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://www.capho.org/sites/default/files/nops/Generic%20Imatinib%20Review%20of%20the%20Literature%20on%20Clinical%20Efficacy%20-%20Mario%20de%20 Lemos.pdf
48. Dylst P, Vulto A, Simoens S. Societal value of generic medicines beyond cost-saving through reduced prices. Expert Rev Pharmacoecon Outcomes Res. 2015;15(4):701-11.
49. Woerkom M, Piepenbrink H, Godman B, Metz J, Campbell S, Bennie M, et al. Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. J Comp Eff Res. 2012;1(6):527-38.
50. Simoens S. International comparison of generic medicine prices. Curr Med Res Opin. 2007;23(11):2647-54.
51. Wouters OJ, Kanavos PG. A comparison of generic drug prices in seven European countries: a methodological analysis. BMC Health Serv Res. 2017;17(1):242.
52. Hill A, Gotham D, Fortunak J, Meldrum J, Erbacher I, Martin M, et al. Target prices for mass production of tyrosine kinase inhibitors for global cancer treatment. BMJ Open. 2016;6(1):e009586.
53. Hill A, Redd C, Gotham D, Erbacher I, Meldrum J, Harada R. Estimated generic prices of cancer medicines deemed cost-ineffective in England: a cost estimation analysis. BMJ Open. 2017;7(1):e011965.
54. Chen CT, Kesselheim AS. Journey of generic imatinib: a case study in oncology drug pricing. J Oncol Pract. 2017;13(6):352-5.
55. Barber M, Gotham D, Hill A. Potential price reductions for cancer medicines on the WHO Essential Medicines List [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://apps.who.int/medicinedocs/documents/s23154en/s23154en.pdf
56. Guan X, Tian Y, Ross-Degnan D, Man C, Shi L. Interrupted time-series analysis of the impact of generic market entry of antineoplastic products in China. BMJ Open. 2018;8(7):e022328.
57. Matusewicz W, Godman B, Pedersen HB, Furst J, Gulbinovic J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):755-8.
58. Hawkes N. Drug company Aspen faces probe over hiking generic prices. BMJ. 2017;357:j2417.
59. Kahn T. Aspen loses Italy appeal over cancer drug prices. Business Day. 2017 Jun 15.
60. Pagliarulo N. EU investigating generic drugmaker over cancer drug price hikes. Biopharma Dive. 2017 May 15.
61. Godman B, Wilcock M, Martin A, Bryson S, Baumgärtel C, Bochenek T, de Bruyn M. Generic pregabalin; current situation and implications for health authorities, generics and biosimilars manufacturers in the future. Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(3):125-35. doi:10.5639/gabij.2015.0403.028
62. McLaughlin M, Kotis D, Thomson K, Harrison M, Fennessy G, Postelnick M, et al. Empty shelves, full of frustration: consequences of drug shortages and the need for action. Hosp Pharm. 2013;48(8):617-8.
63. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198-206.
64. Kosti M, Djakovic L, Šuji R, Godman B, Jankovi SM. Inflammatory Bowel Diseases (Crohn’s disease and Ulcerative Colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85-93.
65. Cameron A, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Switching from originator brand medicines to generic equivalents in selected developing countries: how much could be saved? Value Health. 2012;15(5):664-73.
66. Wild C, Grössmann N, Bonanno PV, Bucsics A, Furst J, Garuoliene K, et al. Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 2016;27(11):2134-6.
67. Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing? Br Clin Pharmacol. 2010;70(3):335-41.
68. Ferner RE, Lenney W, Marriott JF. Controversy over generic substitution. BMJ. 2010;340:c2548.
69. Medicines and Healthcare products Regulatory Agency. Antiepileptic drugs: updated advice on switching between different manufacturers’ products. 2017 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.gov.uk/drug-safety-update/antiepileptic-drugs-updated-advice-on-switching-between-different-manufacturers-products#chm-review-and-update
70. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Policies to enhance prescribing efficiency in Europe: findings and future implications. Front Pharmacol. 2010;1:141.
71. McKee M, Stuckler D, Martin-Moreno JM. Protecting health in hard times. BMJ. 2010;341:c5308.
72. Markovic-Pekovic V, Skrbic R, Godman B, Gustafsson LL. Ongoing initiatives in the Republic of Srpska to enhance prescribing efficiency: influence and future directions. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):661-71.
73. Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, Keuerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PloS One. 2017;12(12):e0190147.
74. Baumgärtel C, Godman B, Malmström R, Andersen M, Abuelkhair M, Abdu S, et al. What lessons can be learned from the launch of generic clopidogrel? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68. doi:10.5639/gabij.2012.0102.016
75. Organisation for Economic Co-operation and Development. OECD Glossary of statistical terms [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://www.oecd-ilibrary.org/docserver/download/3008121e.pdf?expires=1521443673&id=id&accname=guest&checksum=EDB68C374A8FE629D5A2C1FAFB0AEB6C
76. Coma A, Zara C, Godman B, Agusti A, Diogene E, Wettermark B, et al. Policies to enhance the efficiency of prescribing in the Spanish Catalan region: impact and future direction. Expert Rev Pharmacoecon Outcomes Res. 2009;9(6):569-81.
77. Kanavos P. Generic policies: rhetoric vs. reality. Euro Observer. 2008;10(2):1-6.
78. Piperska Group. Piperska 2017 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://www.piperska.org/
79. Garattini S, Bertele V, Godman B, Haycox A, Wettermark B, Gustafsson LL. Enhancing the rational use of new medicines across European health care systems. Eur J Clin Pharmacol. 2008;64(12):1137-8.
80. Moon JC, Godman B, Petzold M, Alvarez-Madrazo S, Bennett K, Bishop I, et al. Different initiatives across Europe to enhance losartan utilization post generics: impact and implications. Front Pharmacol. 2014;5:219.
81. Godman B, Petzold M, Bennett K, Bennie M, Bucsics A, Finlayson AE, et al. Can authorities appreciably enhance the prescribing of oral generic risperidone to conserve resources? Findings from across Europe and their implications. BMC Med. 2014;12:98.
82. Vonina L, Strizrep T, Godman B, Bennie M, Bishop I, Campbell S, et al. Influence of demand-side measures to enhance renin-angiotensin prescribing efficiency in Europe: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):469-79.
83. Ferrario A, Arja D, Bochenek T, ati T, Dankó D, Dimitrova M, et al. The implementation of managed entry agreements in Central and Eastern Europe: findings and implications. Pharmacoecon. 2017;35(12):1271-85.
84. Godman B, Shrank W, Wettermark B, Andersen M, Bishop I, Burkhardt T, et al. Use of generics – a critical cost containment measure for all healthcare professionals in Europe? Pharmaceuticals. 2010;3(8):2470-94.
85. Godman B, Bucsics A, Burkhardt T, Piessnegger J, Schmitzer M, Barbui C, et al. Potential to enhance the prescribing of generic drugs in patients with mental health problems in Austria; implications for the future. Front Pharmacol. 2012;3:198.
86. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
87. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe: a descriptive overview. Health Policy. 2012;104(1):50-60.
88. Leporowski A, Godman B, Kurdi A, MacBride-Stewart S, Ryan M, Hurding S, et al. Ongoing activities to optimize the quality and efficiency of lipid-lowering agents in the Scottish national health service: influence and implications. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):655-66.
89. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.whocc.no/
90. Venkatesan S, Lamfers M, Leenstra S, Vulto AG. Overview of the patent expiry of (non-) tyrosine kinase inhibitors approved for clinical use in the EU and the US. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(2):89-96. doi:10.5639/gabij.2017.0602.016
91. Clopes A, Gasol M, Cajal R, Segu L, Crespo R, Mora R, et al. Financial consequences of a payment-by-results scheme in Catalonia: gefitinib in advanced EGFR-mutation positive non-small-cell lung cancer. J Med Econ. 2017;20(1):1-7.
92. Dylst P, Simoens S. Does the market share of generic medicines influence the price level?: a European analysis. Pharmacoecon. 2011;29(10):875-82.
93. BBC News – Health. Cancer drugs price rise ‘costing NHS millions’. 2017 Jan 29.
94. Vella O. Further reductions in medicine prices. Times of Malta. 2017 Jan 15. Available from: https://www.timesofmalta.com/articles/view/20170115/consumer-affairs/Further-reductions-in-medicine-prices.636591
95. Petrova G. Pricing and reimbursement of medicines in Central and East European countries. J Pharmaceut Res Health Care. 2014;2(6);4311-28.
96. Kawalec P, Stawowczyk E, Tesar T, Skoupa J, Turcu-Stiolica A, Dimitrova M, et al. Pricing and reimbursement of biosimilars in Central and Eastern European countries. Front Pharmacol. 2017;8:288.
97. Baltic Statistics on Medicines 2010-2012 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://apps.who.int/medicinedocs/documents/s21301en/s21301en.pdf
98. Marcheva M T-KL, Petrova G. Pricing and reimbursement of medicines in Central and East European countries. World Journal of Pharmacy and Pharmaceutical Sciences. 2013;2(6):4311-28.
99. WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies. Festøy H, Aanes T, Ognøy AE. PPRI Pharma Profile Norway June 2015 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://ppri.goeg.at/sites/ppri.goeg.at/files/inline-files/PPRI%20Norway%202018.pdf
100. Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11(1):121-9.
101. Kalaba M, Godman B, Vuksanovi A, Bennie M, Malmström RE. Possible ways to enhance renin-angiotensin prescribing efficiency: Republic of Serbia as a case history? J Comp Eff Res. 2012;1(6):539-49.
102. Vogler S, Zimmermann N, Gombocz M, Mörtenhuber S, Skala R, Lichtenecker J, et al. Short PPRI Pharma Profile Austria. January 2018 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://ppri.goeg.at/sites/ppri.goeg.at/files/inline-files/Short_PPRI_Pharma_Profile_AT_2017_final_neu_1.pdf
103. Bucsics A, Godman B, Burkhardt T, Schmitzer M, Malmström RE. Influence of lifting prescribing restrictions for losartan on subsequent sartan utilization patterns in Austria: implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2012;12(6):809-19.
104. Godman B, Abuelkhair M, Vitry A, Abdu S, Bennie M, Bishop I, et al. Payers endorse generics to enhance prescribing efficiency: impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2012.0102.017
105. Pontén J, Rönnholm G, Skiöld P. PPRI Pharma Profile Sweden. April 2017 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://ppri.goeg.at/sites/ppri.goeg.at/files/inline-files/PPRI_Pharma_Profile_Sweden_2017_5.pdf
106. Godman B, Persson M, Miranda J, Skiold P, Wettermark B, Barbui C, et al. Changes in the utilization of venlafaxine after the introduction of generics in Sweden. Appl Health Econ Health Pol. 2013;11(4):383-93.
107. GaBI Online – Generics and Biosimilars Initiative. The Greek problem of generics pricing [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 14]. Available from: www.gabionline.net/Generics/Research/The-Greek-problem-of-generics-pricing.
108. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilar Journal. 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
109. Dylst P, Vulto A, Simoens S. Analysis of the Italian generic medicines retail market: recommendations to enhance long-term sustainability. Expert Rev Pharmacoecon Outcomes Res. 2015;15(1):33-42.
110. Godman B, Bishop I, Finlayson AE, Campbell S, Kwon HY, Bennie M. Reforms and initiatives in Scotland in recent years to encourage the prescribing of generic drugs, their influence and implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):469-82.
111. McGinn D, Godman B, Lonsdale J, Way R, Wettermark B, Haycox A. Initiatives to enhance the quality and efficiency of statin and PPI prescribing in the UK: impact and implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):73-85.
112. Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):125-30.
113. UK Departemnt of Health and Social Care. Health service medical supplies (costs) bill factsheet. Updated 8 November 2016 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.gov.uk/government/publications/health-service-medical-supplies-costs/health-service-medical-supplies-costs-bill-factsheet
114. Bundesministerium für Gesundheit. Preismoratorium für Arzneimittel [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.bundesgesundheitsministerium.de/themen/krankenversicherung/arzneimittelversorgung/preismoratorium.html
115. Bundesministerium für Gesundheit. Sozialgesetzbuch (SGB) Fünftes Buch (V) – Gesetzliche Krankenversicherung – (Artikel 1 des Gesetzes v. 20. Dezember 1988, BGBl. I S. 2477). § 130a Rabatte der pharmazeutischen Unternehmer [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.gesetze-im-internet.de/sgb_5/__130a.html.
116. UK Government. Pharma firms accused of illegal agreements over life-saving drug. 28 February 2019 [homepage on the Internet]. [cited 2019 Mar 14]. Available from: https://www.gov.uk/government/news/pharma-firms-accused-of-illegal-agreements-over-life-saving-drug
117. European Commission. Antitrust: Commission opens formal investigation into Aspen Pharma’s pricing practices for cancer medicines. 2017. Available at URL: file:///C:/Users/mail/Downloads/IP-17-1323_EN.pdf
118. Department of Health. Press Release. Ireland to open negotiations with Belgium, the Netherlands, Luxembourg and Austria on drug pricing and supply – Minister Harris [homepage on the Internet]. [cited 2019 Mar 14]. Available from:http://health.gov.ie/blog/press-release/ireland-to-open-negotiations-with-belgium-the-netherlands-luxembourg-and-austria-on-drug-pricing-and-supply-minister-harris/
119. European Observatory. How can voluntary cross-border collaboration in public procurement improve access to health technologies in Europe? [homepage on the Internet]. [cited 2019 Mar 14]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0009/331992/PB21.pdf?ua=1
120. Ferrario A, Humbert T, Kanavos P, Pedersen HB. Strategic procurement and international collaboration to improve access to medicines. Bull World Health Organ. 2017;95(10):720-2.
121. Vogler S, Zimmermann N, Babar ZU. Price comparison of high-cost originator medicines in European countries. Expert Rev Pharmacoecon Outcomes Res.2017;17(2):221-30.
122. Vogler S, Kilpatrick K, Babar ZU. Analysis of medicine prices in New Zealand and 16 European countries. Value in Health. 2015;18(4):484-92.
123. Bochenek T, Abilova V, Alkan A, Asanin B, de Miguel Beriain I, Besovic Z, et al. Systemic Measures and Legislative and Organizational frameworks aimed at preventing or mitigating drug shortages in 28 European and Western Asian countries. Frontiers in pharmacology. 2017;8:942.
124. Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, et al. Barriers for Access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
|
Author for correspondence: Brian Godman, BSc, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2019 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/pricing-of-oral-generic-cancer-medicines-in-25-european-countries-findings-and-implications.html
Copyright ©2024 GaBI Journal unless otherwise noted.
Strategies for pricing of pharmaceuticals and generics in developing countries
Abstract:
Dr Brian Godman and Professor Mohamed Azmi Hassali review Rida et al. regarding pricing strategies for pharmaceuticals in developing countries.
Submitted: 15 March 2017; Revised: 22 March 2017; Accepted: 24 March 2017; Published online first: 6 April 2017
Rida and Ibrahim are to be congratulated on their extensive review of ongoing pricing strategies in developing countries [1], also referred to as lowerand middle-income countries (LMICs). These include advocating policies regarding markups for pharmaceuticals, pricing formulae for medicines, external reference pricing, as well as encouraging greater use of generics [2–4]. However, there are concerns over external reference pricing (ERP), especially for new medicines where pharmaceutical companies are potentially delaying launch or not launching in some countries to maintain high prices [5]. There are also concerns over the ability to rapidly obtain low prices for generics if prices under ERP systems are only reviewed annually or biannually. Aggressive pricing policies in The Netherlands, including quarterly tendering, led to prices of generic omeprazole and simvastatin dropping to just 2% of the originator price in a short time period [6]. In Sweden, compulsory generics substitution with the lowest priced generic drug also led to rapid price erosion following generic availability. Prices fell further following the instigation of monthly auctions where the cheapest generic drug was guaranteed a substantial proportion of the market the following month [7, 8].
The prices of pharmaceuticals are a particular issue in LMICs, where medicines can account for up to 60% of total healthcare expenditures, and where up to 90% of the population purchase medicines through copayments [9, 10]. High co-payments impact on adherence of non-communicable diseases (NCDs) as well as on access to biologicals to treat immunological diseases [11–13]. This is a concern with NCDs as for example, currently three out of four patients with hypertension live in LMICs [14]. As mentioned by Rida and Ibrahim, promoting generics is a major way to reduce prices and enhance access to appropriate medicines [1, 10, 15, 16]. Although things are changing [10, 16], to date, there have only been a limited number of policy evaluations surrounding generics in LMICs [17]. There is also a comparative lack of strategies in place to combat the activities of pharmaceutical companies who are promoting their branded medicines negatively impacting on the use of generics [18]. The lack of promotion of generics can often be coupled with a lack of formal pricing strategies for generics [8]. This can be a major concern for countries and patients with NCDs, as generics for NCDs can be manufactured and distributed for as little as US$1 per patient per month [19, 20].
The lack of formal pricing strategies in place for generics in LMICs contrasts sharply with European countries where there are multiple formal pricing policies. These can be collated under three main themes [10, 21, 22] and include [8]: regulated systems (prescriptive pricing) where there are established rules for the pricing of generics, as seen in Belgium, Croatia, France, Hungary, Norway and Poland; free pricing – where manufacturers are (relatively) free to set prices of generics, as seen in Germany, The Netherlands, Sweden and the UK; however, typically there are programmes in place to obtain low prices; or a mixed approach – which is a combination of the two different approaches, as currently seen in Austria [10, 23–26].
As a result of the different pricing policies, there can be substantial price differences for generics [8, 22, 27]. Overall, generics prices can vary by up to 36 fold across countries depending on the pricing policies [28], with prices generally lower in countries with higher consumption of generics [29]. This is independent of the size of the country [30, 31]. Different countries across Europe and other parts of the world have also used a variety of other approaches to promote the use of generics. These include educational approaches, financial incentives and laws. Laws include compulsory generics substitution, as seen in Sweden, or compulsory International Nonproprietary Name (INN) prescribing, as seen in Lithuania [7, 30].
Despite the efforts being made, there are still a number of barriers that need to be addressed to enhance the prescribing and dispensing of generics, especially in LMICs [10, 32, 33]. These include addressing fears associated with generics substitution that are enhanced by concerns over the efficacy and safety of generics [34, 35]. Such fears resulted in Hassali et al. developing a list of requirements that should be met to enhance successful substitution [36]. Educational needs include encouraging high INN prescribing which is advocated by the World Health Organization (WHO) for non-controversial products, as seen in Scotland where rates are close to the 100% [8, 37]. Financial incentives include greater patient co-payments for branded products of the same molecule, which is typically practised across Europe [22]. Potential initiatives can also include reducing financial disincentives to the prescribing of generics, as currently seen in China. Here, both hospitals and physicians need to prescribe branded products with associated procured discounts to enhance their incomes [38]. This is in addition to the measures mentioned in Table 1 by Rida and Ibrahim [1].
It is important that appropriate care is taken when introducing pricing policies for generics. In 2012, the South Korean Government set the same maximum reimbursement price for originators and generics in an attempt to make the market more competitive [39], building on earlier reforms mentioned in Table 1 by Rida and Ibrahim [1]. However, given the concerns that still exist among physicians in Korea regarding generics, the opposite was achieved. The price dispersion between different generics significantly decreased and originator utilization significantly increased [39]. A similar situation was seen when compulsory INN prescribing was introduced in Abu Dhabi, United Arab Emirates. Implementation of this policy did not achieve the desired results as physicians were not incentivized to preferentially prescribe the generic medicines and pharmacists’ remuneration was not altered to preferentially dispense the cheapest INN product [40].
Many countries have also implemented a variety of approaches to the pricing and reimbursement of new medicines. Whilst all countries use critical appraisal techniques to assess the level of health gain with new medicines as part of pricing negotiations, some countries use this as a basis for pricing negotiations, e.g. Austria, France and Germany. Others use this information to develop economic para meters, such as the extent of an increase in quality adjusted life years (QALYs) with the new medicine with or without a budget impact analysis [41–43]. One concern is that most countries that utilize QALYs do not set thresholds, which can be exploited by pharmaceutical companies, especially in emotive disease areas, such as cancer and orphan diseases [41, 44, 45].
There is also growing use of risk-sharing arrangements brought about by the everincreasing prices of new medicines and issues of affordability in European countries [42, 46, 47]. This contrasts sharply with LMIC countries where there are currently few formal pricing approaches for new medicines, as discussed by Rida and Ibrahim [1]. Examples of pricing strategies that could be considered by LMICs when funding new medicines include those currently instigated in Austria [42, 48]. This is in addition to ERP as advocated by WHO [4]. In Austria, new medicines that are similar to existing standards are expected to be priced lower than the current standard prices for reimbursement [49]. New medicines that have added benefit can potentially command up to 10% more than the current standard prices, with prices for new medicines that are deemed to have substantially more added value, being allowed to have prices similar to European countries endorsed by health economic evaluations [49]. In the case of LMICs, this would mean prices similar to other LMICs with similar economic situations, as seen in Europe [3]. With only a few new medicines typically seen as innovative, this means that most new medicines will be priced below or just above current standards [42, 48], greatly increasing affordability and access.
In conclusion, Rida and Ibrahim’s research has been extensive. They have highlighted the need for LMICs to develop suitable pricing strategies for medicines to enhance access to affordable medicines. This is essential given rising rates of NCDs and other diseases. There is an opportunity for LMICs to learn from other countries, such as those in Europe who are developing strategies to cope with growing resource pressures on healthcare systems and the need to maintain equitable and comprehensive health care for all [8, 42].
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
Authors
Brian Godman1,2,3, BSc, PhD
Professor Mohamed Azmi Hassali4, PhD
1Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, G4 0RE Glasgow, UK
2Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
3Health Economics Centre, University of Liverpool Management School, Liverpool, UK
4Discipline of Social and Administrative Pharmacy, School of Pharmaceutical Sciences, Universiti Sains Malaysia, MY-11800 Minden, Penang, Malaysia
References
1. Rida NMA, Ibrahim M. Pricing strategies for pharmaceuticals in developing countries: what option do we have? Generics and Biosimilars Journal (GaBI Journal). 2017;6(1):4-6. doi:10.5639/gabij.2017.0601.002
2. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198-206.
3. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe: a descriptive overview. Health Policy. 2012;104(1):50-60.
4. World Health Organization. WHO Guideline on country pharmaceutical pricing policies [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://apps.who.int/medicinedocs/documents/s21016en/s21016en.pdf
5. European Commission. Carone G, Schwierz C, Xavier A. Cost-containment policies in public pharmaceutical spending in the EU [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://ec.europa.eu/economy_finance/publications/economic_paper/2012/pdf/ecp_461_en.pdf
6. Woerkom Mv, Piepenbrink H, Godman B, Metz J, Campbell S, Bennie M, et al. Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. J Comp Eff Res. 2012;1(6):527-38.
7. Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9(1): 65-83
8. Godman B, Baker A, Leporowski A, Morton A, Baumgärtel C, Bochenek T, Fadare J, et al. Initiatives to increase the prescribing of low cost generics; the case of Scotland in the international context. Medical Research Archives. 2017;5(3):1-34
9. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240-9.
10. HAI. Policy options for promoting the use of generic medicines in low and middle-income countries. March 2016 [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://haiweb.org/wp-content/uploads/2017/02/HAI_Review_generics_policies_final.pdf
11. Kostic M, Djakovic L, Šujić R, Godman B, Jankovic SM. Inflammatory Bowel Diseases (Crohn’s Disease and Ulcerative Colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85-93.
12. Choudhry NK, Denberg TD, Qaseem A. Improving adherence to therapy and clinical outcomes while containing costs: opportunities from the greater use of generic medications: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2016;164(1):41-9.
13. Simoens S, Sinnaeve PR. Patient co-payment and adherence to statins: a review and case studies. Cardiovasc Drugs Ther. 2014;28(1):99-109.
14. Irazola VE, Gutierrez L, Bloomfield G, Carrillo-Larco RM, Dorairaj P, Gaziano T, et al. Hypertension prevalence, awareness, treatment, and control in selected LMIC Communities: results from the NHLBI/UHG Network of Centers of Excellence for Chronic Diseases. Glob Heart. 2016;11(1):47-59.
15. Cameron A, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Switching from originator brand medicines to generic equivalents in selected developing countries: how much could be saved? Value Health. 2012;15(5):664-73.
16. Hassali MA, Alrasheedy AA, McLachlan A, Nguyen TA, Al-Tamimi SK, Ibrahim MI, et al. The experiences of implementing generic medicine policy in eight countries: a review and recommendations for a successful promotion of generic medicine use. Saudi Pharm J. 2014;22(6):491-503.
17. Kaplan WA, Ritz LS, Vitello M, Wirtz VJ. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000-2010. Health Policy. 2012;106(3):211-24.
18. Civaner M. Sale strategies of pharmaceutical companies in a “pharmerging” country: the problems will not improve if the gaps remain. Health Policy. 2012;106(3):225-32.
19. Novartis. Kenya first country to launch ‘Novartis Access’, expanding affordable treatment options against chronic diseases. 15 Oct 2015. Available from: https://www.novartis.com/news/media-releases/kenya-first-country-launch-novartis-access-expanding-affordable-treatment
20. Novartis. Novartis access. Available from: https://www.novartis.com/about-us/corporate-responsibility/expanding-access-healthcare/novartisaccess
21. Godman B, Shrank W, Wettermark B, Andersen M, Bishop I, Burkhardt T, et al. Use of genericsa critical cost containment measure for all healthcare professionals in Europe? Pharmaceuticals (Basel). 2010;3(8):2470-94.
22. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilar Journal. 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
23. Sermet C, Andrieu V, Godman B, Van Ganse E, Haycox A, Reynier JP. Ongoing pharmaceutical reforms in France: implications for key stakeholder groups. Appl Health Econ Health Policy. 2010;8(1):7-24.
24. Dylst P, Vulto A, Simoens S. Analysis of French generic medicines retail market: why the use of generic medicines is limited. Expert Rev Pharmacoeco Outcomes Res. 2014;14(6):795-803.
25. Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11(1):121-9.
26. Brkičić LS, Godman B, Bogut M, Sršen M, Kwon H-Y, de Bruyn W, Tabain T. Pharmaceutical pricing in Croatia: a comparison of ordinances in 2013 versus 2009 and their potential savings to provide future guidance. Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(2):79-89. doi:10.5639/gabij.2015.0402.017
27. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
28. Simoens S. International comparison of generic medicine prices. Curr Med Res Opin. 2007;23(11):2647-54.
29. Dylst P, Simoens S. Does the market share of generic medicines influence the price level?: a European analysis. Pharmacoeconomics. 2011;29(10):875-82.
30. Garuoliene K, Godman B, Gulbinovič J, Wettermark B, Haycox A. European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
31. Markovic-Pekovic V, Skrbić R, Godman B, Gustafsson LL. Ongoing initiatives in the Republic of Srpska to enhance prescribing efficiency: influence and future directions. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):661-71.
32. Nguyen TA, Hassali MAA, McLachlan A. Generic medicines policies in the Asia Pacific region: ways forward. WHO South-East Asia Journal of Public Health. 2013;2(1):72-4.
33. Fadare JO, Adeoti AO, Desalu OO, Enwere OO, Makusidi AM, Ogunleye O, et al. The prescribing of generic medicines in Nigeria: knowledge, perceptions and attitudes of physicians. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):639-50.
34. Kumar R, Hassali MA, Saleem F, Alrasheedy AA, Kaur N, Wong ZY, et al. Knowledge and perceptions of physicians from private medical centres towards generic medicines: a nationwide survey from Malaysia. J Pharm Policy Pract. 2015;8(1):11.
35. Hassali MA, Wong ZY, Alrasheedy AA, Saleem F, Mohamad Yahaya AH, Aljadhey H. Perspectives of physicians practicing in low and middle income countries towards generic medicines: a narrative review. Health Policy. 2014;117(3):297-310.
36. Hassali MA, Thambyappa J, Saleem F, ul Haq N, Aljadhey H. Generic substitution in Malaysia: recommendations from a systematic review. J Appl Pharm Sci. 2012;2(8):159-64.
37. Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015). BMC Public Health. 2016;16:724.
38. Zeng W, Zhen J, Feng M, Campbell SM, Finlayson AE, Godman B. Analysis of the influence of recent reforms in China: cardiovascular and cerebrovascular medicines as a case history to provide future direction. J Comp Eff Res. 2014;3(4):371-86.
39. Kwon HY, Kim H, Godman B, Reich MR. The impact of South Korea’s new drug-pricing policy on market competition among off-patent drugs. Expert Rev Pharmacoecon Outcomes Res. 2015;15(6):1007-14.
40. Abuelkhair M, Abdu S, Godman B, Fahmy S, Malmström RE, Gustafsson LL. Imperative to consider multiple initiatives to maximize prescribing efficiency from generic availability: case history from Abu Dhabi. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):115-24.
41. Organisation for Economic C-operation and Development. Paris V. Belloni A. Value in pharmaceutical pricing [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://www.oecd-ilibrary.org/social-issues-migration-health/value-in-pharmaceutical-pricing_5k43jc9v6knx-en
42. European Parliament. Godman B, Ortwijn W, de Waure C, Mosca I, Puggina A, Specchia ML et al. Links between pharmaceutical R&D models and access to affordable medicines. A study for the ENVI Committee [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://www.europarl. europa.eu/RegData/etudes/STUD/2016/587321/IPOL_STU(2016)587321_EN.pdf
43. Faleiros DR, Alvares J, Almeida AM, de Araújo VE, Andrade EI, Godman BB, et al. Budget impact analysis of medicines: updated systematic review and implications. Expert Rev Pharmacoecon Outcomes Res. 2016;16(2):257-66.
44. Simoens S, Picavet E, Dooms M, Cassiman D, Morel T. Cost-effectiveness assessment of orphan drugs: a scientific and political conundrum. Appl Health Econ Health Policy. 2013;11(1):1-3.
45. Haycox A. Why cancer? Pharmacoeconomics. 2016;34(7):625-7.
46. Ferrario A, Kanavos P. Dealing with uncertainty and high prices of new medicines: a comparative analysis of the use of managed entry agreements in Belgium, England, the Netherlands and Sweden. Soc Sci Med. 2015;124:39-47.
47. Ferrario A, Kananvos P. Managed entry agreements for pharmaceuticals: the European experience. EMiNet, Brussels, Belgium. Available from: http://eprints.lse.ac.uk/50513/2013
48. World Health Organization. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research. [homepage on the Internet]. [cited 2017 Mar 22]. Available from: http://www.euro.who.int/_data/assets/pdf_file/0008/306179/Access-new-medicines-TR-PIO-collaboration-research.pdf?ua=1
49. Godman B, Bucsics A, Burkhardt T, Haycox A, Seyfried H, Wieninger P. Insight into recent reforms and initiatives in Austria: implications for key stakeholders. Expert Rev Pharmacoecon Outcomes Res. 2008;8(4):357-71.
Author for correspondence: Brian Godman, BSc, PhD, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, G4 0RE Glasgow, UK; Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186 Stockholm, Sweden
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2017 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/strategies-for-pricing-of-pharmaceuticals-and-generics-in-developing-countries.html