

Author byline as per print journal: Ka-Liong Tan1, DPhil; Ainoon Othman1; Irwan Mohd Subri2,3; Noor Fadzilah Zulkifli1; Mohd Mahyeddin Mohd Salleh3; Nazariyah Yahaya4; Khairun Nain Nor Aripin1; Shahirah Nadiah Shaharuddin5; Seri Azalina Mohd Ghazalli6; Muhammad Syazan Sulaiman6
|
In recent years, there has been a rapid growth of the halal pharmaceutical industry, especially in the supply chain of solid oral dosage forms of medication. This article outlines aspects of the Halal Management System (HMS) in the development and production of halal pharmaceuticals. It explains the needs and requirements of HMS and identifies the challenges faced in implementation. The article outlines aspects of execution and hurdles encountered when standardizing halal certification. The article also highlights the need for systematic traceability systems and effective product recall mechanisms to ensure adherence to halal requirements. It also highlights the grey areas for halal in terms of pharmaceutical manufacture that are brought about by use of non-halal raw materials, e.g. alcohol, gelatine, glycerin, lecithin, glutamic acid and stearates. |
Submitted: 4 April 2023; Revised: 22 August 2023; Accepted: 23 August 2023; Published online first: 5 September 2023
Halal is an integral observance for all Muslims. This concept originates from an Arabic word and can be defined as permissible by shariah law. The consumption of halal foods is mandated under Islamic teachings and includes, water and beverages, meals and snacks, as well as pharmaceutical medicines. Medicines also fall under Islamic dietary law and are required to be halal and Muslims are forbidden to use illicit drugs except in an emergency [1].
In recent years, the global demand for halal pharmaceuticals has been increasing. This comes with the growth of the global population of Muslims which is expected to grow from 1.6 billion to 2.2 billion by 2030 [2]. It is estimated that halal pharmaceuticals now account for 22% of the total value of all halal products [3]. Furthermore, halal pharmaceuticals have also gained increasing acceptability among non-Muslims due to ethical consumption issues such as social responsibility, stewardship, economic and social justice, animal welfare, as well as ethical investment. In addition, many pharmaceutical companies are aspiring towards bigger investment and development of halal-certified products.
The term pharmaceutical refers to both prescription and non-prescription medicinal products in finished dosage forms, i.e. biopharmaceuticals, radiopharmaceuticals, and traditional medicines. The dosage forms can be administered via oral intake, through body orifices, given as injections, implants, or through topical application [4]. Common orally administered drugs can be in the forms of tablets, soft-gel capsules, chewable, orally disintegrating tablets, sublingual tablets, capsules, lipids, and powder. Table 1 shows the classification of oral dosage forms available on the market. According to a recent survey, oral dosage forms are the most popular means of delivering active pharmaceutical ingredients (API) to patients [5]. Oral dosage forms provide better protection against moisture, oxygen, and light before the medication is consumed and released into the body as compared to injectables and topical formulations. Halal pharmaceuticals are better outlined in the supply chain and Table 2 lists the halal-related standards available in Malaysia.
The HMS is an approach used in the detection of non-halal contaminations which incorporates control steps to the production process to ensure that products and services are halal and safe. HMS is crucial to regulate non-halal elements and safeguard the integrity of halal products and services. It covers all aspects of sourcing, manufacturing procedure, packaging, and logistics, see Figure 1. The system provides both guidelines for hazard analysis critical control point (HACCP) and good manufacturing practice (GMP) in product processing to ensure compliance with shariah requirements. Overall, when it comes to halal pharmaceutical production, processes must adhere to local and international standards including: GMP, good hygiene practice (GHP), good clinical practice (GCP), good laboratory practice (GLP), good storage practice (GSP), good engineering practice (GEP), and good distribution practice (GDP). In view of the complexity of HMS, more efforts are required for stakeholders and regulators of the pharmaceutical industry worldwide to understand these features and ensure the adherence of halal-pharmaceuticals.
Medicines are often prescribed in emergencies to treat conditions that may be life-threatening, thus the issue of whether the product is halal issue is not always the highest priority. This can partly explain why the halal pharmaceutical industry remains in a nascent stage. In the absence of strong market demand, halal pharmaceuticals need to rely on governments and industry players in Muslim-dominant countries to promote awareness. Suppliers fail to prioritize the halal certification of pharmaceuticals and, to address this challenge, some experts recommend that governments and manufacturers implement the relevant halal management and accreditation processes themselves.
To implement halal management, continuous social science research, clinical experiments, and development initiatives are necessary to identify alternatives to non-halal ingredients. Established international halal regulations are now gaining more recognition, especially those concerning oral dosage forms.
The growth of the halal supply chain to produce solid oral dosage pharmaceuticals presents various challenges. Despite halal’s increasing popularity by both Muslims and non-Muslims, there is a lack of understanding about the halal concept, leading to a poor recognition of its requirements in pharmaceutical preparation. The complexity of ingredients and processes involved makes the implementation of HMS challenging. Non-compliance with halal regulations can result in product recalls, emphasizing the need for systematic traceability and resource utilization to minimize costs during such a crisis. Furthermore, the lack of Muslim human capital in the industry poses an additional obstacle to sustainable HMS implementation. Therefore, there is a need to review the disputes and challenges faced by the implementation of HMS, focusing on the manufacturing of solid oral dosage forms.
This article aims to provide justification for the implementation of HMS in the manufacturing of solid oral dosage forms by the pharmaceutical industry. The article also provides some solutions and strategies to improve compliance with halal regulations.
International organisations, such as the World Halal Council (WHC) and the Standards and Metrology Institute for Islamic Countries, work together to oversee the standardization of halal certification and accreditation processes. Here, stakeholders actively participate in the development process through working groups and public comments [6]. Several countries, including South Korea and ASEAN (Association of Southeast Asian Nations) have set up national halal certification policies in accordance with International Standards Organization (ISO). The development process also complies with the guidelines established by the World Trade Organization (WTO). Table 3 lists the halal certification authorities in Southeast Asia.
HMS is the primary industry standard used to maintain the halal status of products. Table 4 depicts the structure of HMS. The halal procedure is key to ensure that all halal products are produced responsibly [7]. The HMS clauses are established to manage the overall quality of an organization in accordance with halal requirements [8]. The certification process may vary based on individual national policies. In Malaysia, medium and large industries can only get halal certification by implementing the complete Halal Assurance System (HAS) [9]. However, small and micro enterprises can obtain the certification via the creation of an Internal Halal Committee (IHC) in accordance with the Malaysian HMS and the Manual Procedure for Malaysian Halal Certification [10-12]. IHC is responsible for designing, monitoring, and assuring the implementation of the six principles of HAS as shown in Table 5. It should consist of at least four members, namely two Muslims at the managerial level, one involved in the acquiring and sourcing process, and the halal executive who is responsible for monitoring the halal affairs of the company [13]. Figure 1 shows an example of the IHC composition.
Regarding halal pharmaceuticals, it is key that guidelines are present to standardize the halal requirements. In Malaysia, this guideline (MS 2424:2019 Halal pharmaceuticals &ndash General requirements) was developed for the production and handling of halal medicines to ensure that pharmaceutical companies comply with shariah requirements. Furthermore, the manufacturers must also establish a dedicated processing line for halal pharmaceuticals.
Figure 3 illustrates the steps of HAS implementation. All materials applied in the manufacturing of halal pharmaceuticals, including API, must comply with halal principles. Materials acquired from suppliers under contracts, any other commercial arrangement, or made in-house must also be subjected to the same requirement. More importantly, all materials must be najs-free, i.e. halal (as described in section 4.0). ‘Najs’ is an Arabic term that means ‘filth’ and is considered non-permissible for consumption according to Islamic law. Finally, companies must use dedicated vehicles with appropriate hygiene and sanitation condition for the transportation of all medicines.
It is key to highlight that, to achieve stainable production of halal pharmaceuticals, manufacturers should employ management and operational team personnel to monitor, identify, record, and report any problems in the halal process based on international standards. This can minimize the risk of contamination, mix-ups, and errors in the production processes, thus protecting consumers from potential risks of sub-standard medicines. Pharmaceutical manufacturers applying for halal certification for their products should comply with all the safety and hygiene requirements and adhere to the requirements of shariah law. Dedicated equipment must be used to avoid cross-contamination of halal by non-halal products [14]. In the cleansing procedure, the equipment must be cleaned using clay to remove any microbial contamination, followed by washing, spraying, and rinsing. Segregation of halal and non-halal products at every stage is obligatory, including storing, displaying, and transporting [15]. Furthermore, primary packaging materials must be customized to prevent contamination post-production. The origin and nature of the paper or plastic packaging, inks, films, and glue are also of concern for halal status. On the packaging, information incorporating the name, brand, minimum content in metrics, name and address of manufacturer/distributor, list of ingredients, code number representing production batch, manufacturers, as well as expiry dates must be outlined.
With the increasing interest in the halal pharmaceutical market, several problems have emerged, such as non-compliance with halal standards and shariah law and the fraudulent use of halal logo and terms. For instance, a halal product was found to be contaminated with non-halal content, i.e. a pig’s DNA, and certified using a fake halal logo, resulting in the suspension of halal certification for the product [16]. This highlights the importance of a credible system to safeguard the integrity of halal products. For the pharmaceutical industry, a systematic traceability system used by manufacturers is essential to sustain HMS. Table 6 lists the known halal non-conformance cases in pharmaceutical establishments. These incidents are commonly detected by authorities when conducting audit checks.
The halal traceability framework was established to maintain the integrity of halal products and ingredients throughout the production and supply chain [17]. The IHC and HAS been put in place to facilitate recall procedures for any products that have been recognized as non-compliant [17]. In Malaysia, the National Pharmaceutical Regulatory Agency (NPRA) regulates halal products, manufacturing plants, and work methods, to expand the production and supply of halal products for the global market. The traceability HMS requirement is in tandem with the existing international standards, i.e. the Pharmaceutical Inspection Co-operation Scheme (PIC/S) GMP Guide. This document is also adhered to by the NPRA. The existing traceability system requirements outlined in the PIC/S standard facilitate the implementation of HMS. With outlined traceability practices in place, minimal integration is needed for the adoption of the HMS guideline. Table 7 compares the traceability for HAS, HACCP and GMP.
Product recall management is the final step in traceability systems. In general, this refers to the process of removing defective medicines from the supply chain, ideally before they reach consumers. For example, these will be products that may cause illness or harm, i.e. unsafe food products, products with potentially adverse effects, or contaminated pharmaceutical products. All manufacturers are required to have effective product recall management systems in place to ensure consumer safety. This is also key for halal products and for ensuring their halal status. A product recall can damage the reputation and financial standing of a company. As a damage control strategy, companies must have a good recall management system in place that include checks for halal compliance.
During a recall crisis, a systematic traceability system and efficient utilization of resources can cut down cost. For example, an individual transportation network is vital to reduce transportation costs and to ensure the halal status during transportation. Overall, preventing the occurrence of product recalls will lead to a high level of customer confidence in halal integrity in the halal industry, particularly in terms of halal assurance.
Overall, there is increasing awareness among consumers, health experts, and various organizations of the need for solid oral dosage formulations that are safe, efficacious, high quality, hygienic, and compliant with religious obligations. More pharmaceutical manufacturers are exploring new values in their production process, especially in terms of identifying suitable alternatives that can substitute non-halal ingredients in the process of halal pharmaceutical production, see Table 8.
To achieve that, comprehensive scientific knowledge of all aspects of pharmaceuticals, including production, transport, and storage is a prerequisite to support halal requirements. Ideally, pharmaceuticals should be developed through various research and development programmes that will facilitate the creation of more alternatives for non-halal ingredients in halal pharmaceutical production. These include intoxicants, pork and its by-products, the meat of dead animals, and blood. Muslims are also prohibited from consuming animals that are grouped as carnivores and predatory birds such as dogs, tigers, owls, and hawks. These ingredients are termed &ndash najs and are thought to be ritually unclean. Despite the criteria for halal being fairly clear, halal pharmaceuticals do present some grey areas in terms of manufacturing processes. Below is a summary of a number of components of pharmaceuticals that are not halal compliant.
Alcohol
The use of alcohol in producing API. Although the use of ethanol derived from the manufacturing of alcoholic beverages is not allowed, the use of alcohol compounds as processing aids and stabilising agents is permissible as its trace amount in the final product (0.01%v/v) will not be intoxicating [18].
Gelatine
A long-standing issue affecting Muslim consumers is the use of gelatine from pigs (porcine) and cows (bovine) that are not slaughtered according to Islamic shariah law [19]. With the rise in demand as well as religious concerns surrounding gelatine, there is a need to search for affordable, abundant, and sustainably accessible alternatives [20].
One potential alternative to conventional gelatine (non-halal porcine and bovine) is to ensure that gelatine is derived from animal waste produced in Muslim countries [21]. It is estimated that about 24% of all gelatine originates from bovine and other cattle waste products [22]. Ovines, i.e. sheep and goats are other mammalian sources to extract gelatine [23]. In addition, gelatine derived from chicken by-products can also be considered and used as there is a large amount of chicken waste that includes chicken skin, bones, and shanks. Lastly, fish-sourced gelatine is acceptable to people of all religions.
Glycerine
Glycerine, also referred to as glycerin or glycerol, is used in various products including cosmetics, pharmaceuticals, and foods. Glycerine sourced from animal fat needs to be regulated under the shariah rulings of slaughters to be considered halal. It is not acceptable if the ingredient is extracted from animals that are alive. On the other hand, Halal glycerine can be derived from plants such as palm oils and soybeans.
Lecithin
Lecithin is a fatty substance that occurs naturally in the body tissues of humans, animals, and plants. It can be found naturally in soybeans and yolk. However, it is considered non-halal if obtained from the fatty tissues of non-slaughtered animals. Lecithin functions as an emulsifier by suspending fats and oils to prevent mixing with other substances. It has a variety of medicinal and commercial uses with extensive health benefits. Lecithin supplements are often prescribed to supplement the treatment of high cholesterol, ulcerative colitis, and Alzheimer’s disease.
Glutamic acid
Glutamic acid is an α-amino acid involved in the production of proteins of all living things. Pharmaceutically, glutamic acid supplements have been used to treat behavioural problems and as a supportive treatment of cognitive diseases. It has also been prescribed to prevent nerve damage in chemotherapy patients. In addition, it is also widely used in a variety of cosmetic products.
Glutamic acid can be found naturally in poultry, fish, and all high-protein foods. However, glutamic acid from non-slaughtered animals and pigs are not acceptable for use in halal products.
Disputes of the use and non-halal nature of these components of pharmaceuticals have sparked disputes and constructive discussions in the industry for a long time. The labelling of gelatine, glycerine, lecithin is compulsory to ensure its source of origin is clearly stated. The relevant authorities ensure that all related activities for the manufacturing and handling of halal pharmaceuticals are properly recorded. All businesses must maintain halal control points to ensure that chemicals, reagents, equipment, and other necessities are approved as halal [24]. To date, the scarcity of information remains a key constraint when it comes to the global halal pharmaceutical industry. Most suppliers are not aware of the opportunities in local and global markets. They are also not well versed in the importance of halal certification. In addition, most consumers also have a low level of concern about the content of medications and their halal status. Currently, there is no obligation for the clinics or pharmacy departments to label the micro packaging of medicine. As such, Muslim patients who are not informed of the presence of non-halal materials in the drugs would have unknowingly consumed the prescribed medicinal goods instead of sourcing halal substitutes of the same medicines. This factor contributes to the narrow exploration of the halal market and the difficulty that pharmaceutical manufacturers face in sourcing halal ingredients. The creation of a larger demand from the Muslim population is essential to sensitize the involved stakeholders and push them to undertake more halal product development.
Moreover, at present, the lack of human capital continues to act as a deterrent to the development of the halal pharmaceutical industry. The production of medicines with desirable pharmaceutical qualities that also satisfy religious obligations requires the participation of all involved parties in the manufacturing process. While the halal pharmaceutical industry is growing globally, there is still a lack of understanding of the halal concept, with most of the population associating it with religious matters. Even though religion plays a significant role in halal pharmaceuticals, more awareness about halal products should be instilled to facilitate their widespread production and uptake. Halal education programmes should be developed to educate the producers and the public about the role of halal pharmaceuticals in providing healthy, hygienic, and safe pharmaceutical drugs.
This article has outlined that HMS is an extension of HACCP and GMP guidelines in oral dosage processing to ensure compliance with shariah requirements. It highlights that it is important for manufacturers to uphold their moral commitments and safeguard the concerns of consumers when it comes to halal. This will allow local and international halal commerce to flourish. Non-compliance with halal regulations can result in oral dosage product recall. To reduce the cost during recall, a systematic traceability system is vital. Good HMS implementation can assure the quality and safety of oral dosages to embolden the trust and confidence of patients toward halal pharmaceutical products.
Contributors: We thank Maria Arshad for technical editing the manuscript for grammar and syntax. This manuscript underwent proofreading service by Proofreading by A UK PhD (Registration: NS0163592-K).
This work was financially supported by the Duopharma R&D fund (A2-5-21-804111-16).
KL Tan, A Othman, I Mohd Subri, NF Zulkifli, MM Mohd Salleh, N Yahaya, and KN Nor Aripin are affiliated to Universiti Sains Islam Malaysia (USIMs). SN Shaharuddin is affiliated to Kolej Universiti Islam Perlis (KUIP). All received consultation fee from Duopharma Biotech Berhad, Malaysia.
Competing interests: The authors have no declared conflicts of interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ka-Liong Tan1
Ainoon Othman1
Irwan Mohd Subri2,3
Noor Fadzilah Zulkifli1
Mohd Mahyeddin Mohd Salleh3
Nazariyah Yahaya4
Khairun Nain Nor Aripin1
Shahirah Nadiah Shaharuddin5
Seri Azalina Mohd Ghazalli6
Muhammad Syazan Sulaiman6
1Faculty of Medicine and Health Sciences, University Sains Islam Malaysia (USIM), Persiaran Ilmu, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
2Institute of Fatwa and Halal (IFFAH), University Sains Islam Malaysia (USIM), Persiaran Ilmu, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
3Faculty of Syariah and Law, University Sains Islam Malaysia (USIM), Persiaran Ilmu, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
4Faculty of Science and Technology, University Sains Islam Malaysia (USIM), Persiaran Ilmu, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia
5Faculty of Islamic Studies, Kolej Universiti Islam Perlis (KUIPs), Kuala Perlis, 02000, Perlis.
6Duopharma Biotech Berhad, Suite 18.06, Level 18, CIMB HUB, No. 26, Jalan Sultan Ismail, 50250 Kuala Lumpur
References
1. Al-Fatih S, Esfandiari F. Halal food in South East Asia: are we looking forward? 2020: 166-169. doi: 10.2991/aebmr.k.200226.034
2. Norazmi MN, Lim LS. Halal pharmaceutical industry: opportunities and challenges. Trends Pharmacol Sci. 2015:36(8):496-7.
3. Mohezar S, Zailani S, Tieman M. Tapping into the halal pharmaceutical market: issues and challenges. In: Contemporary issues and development in the global halal industry. Selected Papers from the International Halal Conference 2014. Springer Singapore. 2017. p. 531-41.
4. Zarif MM, Yusof AF. The use of forbidden materials in medicinal products: an Islamic perspective. Middle-East Journal of Scientific Research 13. Approaches of Halal and Thoyyib for Society, Wellness and Health. 2013. doi:10.5829/idosi.mejsr.2013.16.s.10022
5. Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharm Res. 2017:34(5):890-917.
6. Jais AS. Halal related Malaysian standards. Halal Note Series-Halal Common. 2019;1.
7. Perdani CG, Chasanah NU. Evaluation of halal assurance system (HAS) implementation on bakery products processing in small and medium enterprises (case study in X Bakery Batu, East Java). IOP Conference Series: Earth and Environmental Science. 2018:131(1):012023.
8. Evans JR, Lindsay WM. The management and control of quality. Thomson/South-Western; 2005.
9. Idris I, Alias SS, Singh SK. Perception of muslim consumers towards halal branding in advertising. Int J Criminol Sociol. 2020.
10. Jamaludin MA. Fiqh Istihalah.integration of science and Islamic law. Revelation and Science. 2012;2(2):117-23.
11. Salleh AS, Romli F, Salleh KM, Adnan A. Role of Internal Halal Committee in ensuring business sustainability: the case of a multinational slaughterhouse. J Bus Manag Account. 2020:10(2):57-65.
12. Abdullah MS, Noordin MI, Ismail SI, Mustapha NM, Jasamai M, Danik MF, et al. Recent advances in the use of animal-sourced gelatine as natural polymers for food, cosmetics and pharmaceutical applications. Sains Malaysiana. 2018;47(2):323-36.
13. Riaz MN, Chaudry MM. General guidelines for halal food production. In: Handbook of halal food production. CRC Press; 2018. p. 17-28.
14. Di Foggia G, Ferrari S, Lazzarotti V, Pizzurno E. Innovation process for Halal product development: an empirical analysis of Italian firms. Manag Res Pract. 2011: 3(1):27-47.
15. Lam Y, Alhashmi SM. Simulation of halal food supply chain with certification system: a multi-agent system approach. In: Intelligent Agents and Multi-Agent Systems: 11th Pacific Rim International Conference on Multi-Agents. Berlin, Heidelberg: Springer; PRIMA 2008; p. 259-266. doi:10.1007/978-3-540-89674-6_29
16. Majdina Nordin FN, Jasimah Wan Mohamed Radzi CW. Religion and cosmetics: guidelines for preparing products aimed at the Muslim world based on the interpretation of halal cosmetics in Malaysia. J Cosmet Sci. 2021;72(2):139-54.
17. Zainuddin N, Saifudin AM, Deraman N, Osman AA. The effect of halal traceability system on halal supply chain performance. Int. J Sup. Chain Mgt. 2020;9(1):490-8.
18. Maizirwan M, MS Hamzah. Halal issues in pharmaceutical products: urgent need to have modern and efficient production of pharmaceuticals and biopharmaceuticals. Halal Pages. 2010:56-63.
19. Morrison NA, Clark RC, Chen YL, Talashek T, Sworn G. Gelatin alternatives for the food industry. In: Nishinari K, editors. Physical chemistry and industrial application of gellan gum. Berlin, Heidelberg: Springer; 1999. p. 127-31.
20. Alzeer J, Hadeed KA. Halal certification of food, nutraceuticals, and pharmaceuticals in the Arab world. Handbook of healthcare in the Arab world. 2021:765-87. doi:10.1007/978-3-319-74365-3_36-1
21. Alao BO, Falowo AB, Chulayo A, Muchenje V. The potential of animal by-products in food systems: production, prospects and challenges. Sustainability. 2017:9(7):1089.
22. Uddin SM, Hossain MM, Sagadevan S, Al Amin M, Johan MR. Halal and Kosher gelatin: Applications as well as detection approaches with challenges and prospects. Food Bioscience. 2021:44:101422.
23. Abdullah MS, Noordin MI, Ismail SI, Mustapha NM, Jasamai M, Danik MF, et al. Recent advances in the use of animal-sourced gelatine as natural polymers for food, cosmetics and pharmaceutical applications. Sains Malaysiana. 2018;47(2):323-36.
24. Department of Islamic Development Malaysia (Jabatan Kemajuan Islam Malaysia), Malaysia Halal Management System (MHMS) 2020 [homepage on the Internet]. [cited 2023 Aug 22]. Available from: https://smarthalal.com.my/manual.php
|
Author for correspondence:Ka-Liong Tan, DPhil, Faculty of Medicine and Health Sciences, University Sains Islam Malaysia (USIM), Persiaran Ilmu, Putra Nilai, 71800 Nilai, Negeri Sembilan, Malaysia |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2023 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/the-incorporation-of-the-halal-management-system-hms-by-the-pharmaceutical-industry.html
|
Introduction/Study objective: This paper aims to survey the policies implemented by European countries for pricing and promoting the use of biosimilar medicines and to explore similarities and differences with policies for generic medicines. |
Submitted: 30 March 2017; Revised: 26 May 2017; Accepted: 26 May 2017; Published online first: 9 June 2017
Public payers are concerned with ensuring patient access to medicines, particularly in the light of increasing pressure on budgets and the market entry of new, high-priced medicines [1]. One opportunity to generate savings and thus free resources for further investments in health is increased uptake of lower-priced medicines, such as generics. The use of generic medicines has been recommended by the World Health Organization (WHO) [2] and policymakers employ a range of supply- and demand-side tools to increase their uptake. These include generics substitution, physicians prescribing by the International Nonproprietary Name (INN) rather than the brand name, a reference price system, i.e. fixed reimbursement within a cluster of identical and similar medicines, and awareness-raising campaigns [3–9]. The ability of generics-promoting policies to reduce medicine prices and generate savings for health care has been well documented [10, 11].
Since biological medicines also significantly contribute to the pharmaceutical bill, policymakers are awaiting the entry of biosimilar medicines [12, 13], which are expected to generate substantial savings [14]. Recent years have seen several examples of tendering for biosimilar medicines successfully reducing prices [15, 16].
Under the Platform on Access to Medicines in Europe of the Corporate Social Responsibility Process, a multi-stakeholder working group was dedicated to biosimilar medicines. The working group produced a European Commission Consensus Information Document agreed by all stakeholders represented, the document provided key information about biosimilar medicines in order to foster stakeholders’ understanding of biosimilars [17]. However, the working group did not investigate which pricing and usage-enhancing policies European Union Member States applied for biosimilar medicines.
While there is good evidence of the implementation of pricing and demand-side measures for generics in Europe [3–11], the policies that European countries have been implementing to deal with biosimilar medicines are comparatively less known. To the best of the authors’ know ledge, there are few studies in the literature that provide information about pricing and demand-side policies for biosimilar medicines and the only comparative exercise performed across a large number of countries was published by the European Biopharmaceutical Enterprises in 2015 [18]. While the authors recognize the importance of this study, it did not fully explore all aspects of biosimilar medicines policies. Further studies that investigate biosimilar medicines policies are limited to a few countries [19–21] and/or to a single policy [22]. Furthermore, the findings of these studies differ.
Against this backdrop, this manuscript aims to survey the pricing and usage-enhanced policies that different countries, in particular in the European region, have implemented for biosimilar medicines, and to explore whether these policies differ from generic medicines policies.
We conducted a survey with the members of the Pharmaceutical Pricing and Reimbursement Information (PPRI) network [23]. This is a network of competent authorities for pharmaceutical pricing and reimbursement in 46 countries, thereof 43 European countries. It should be noted that European countries are those as defined by WHO [24], and thus include countries such as Israel, Kazakhstan and Kyrgyzstan.
We prepared questions about the status of generic and biosimilar medicines policies. We explored the pricing policies for generics and biosimilars, in particular regarding possible regulation linking the generic and/or biosimilar price to the originator price. We surveyed whether INN prescribing and substitution by generic and biosimilar medicines was permitted, and whether it was mandatory. We also aimed to identify further specific pricing policies, e.g. tendering.
While the focus of this survey was on policies for biosimilar medicines, we also aimed to survey, or validate, information on gen eric medicines policies in order to explore possible differences between policies for the two medicine groups.
As far as possible, we pre-filled the questionnaire with information available to us, through previous research and literature review. This was predominantly only possible in the field of generics. Respondents from the competent authorities were invited to provide, or validate, information on biosimilar and generic medicines policies valid in the first quarter of 2016.
We sent the survey to the PPRI network members on 7 January 2016, requesting their responses by 19 January 2016. A friendly reminder was sent before the deadline, and a further personalized reminder that was focused on key questions was sent on 11 February 2016. Preliminary results were presented and discussed during a meeting with PPRI network members on 28 April 2016, where any misunderstandings could be clarified. In response to this discussion, we created a revised version of the questionnaire, which was circulated for validation on 30 May 2016. During the survey, respondents were encouraged to reply and clarification was sought in the case of answers that raised additional questions. On 1 August 2016, the survey was officially closed and the results were shared with participants. An uncompleted version of the revised questionnaire, i.e. without pre-filled answers, is available in the Annex.
While this survey with the PPRI network was the key survey tool, where considered appropriate we added relevant information from the literature (indicated by references).
Response rate
We received responses from 36 of the 43 European PPRI members, as well as Canada and South Africa. Replies from the European region were provided by 25 of the 28 EU Member States (no data received from Ireland, Italy and Luxembourg) plus Albania, Belarus, Iceland, Israel, Kazakhstan, Kyrgyzstan, Norway, Russia, Serbia, Turkey and Ukraine. Data from the missing three EU Member States and Switzerland were added, wherever possible, from literature and previous PPRI network queries on related topics. As a result, this manuscript includes information from 40 European countries, Canada and South Africa.
Pricing policies
Several countries apply a pricing policy that sets the price of follower products in relation to the price of the originator medicine. For generics, this is called ‘generic price link’. It is a commonly used practice that is applied in 30 of the 42 surveyed countries. Fifteen countries reported that they also apply such a strategy for biosimilar medicines. These are Austria, the Baltic States, Croatia, the Czech Republic, France, Iceland, Italy, Kazakhstan, Norway, Portugal, Romania, Slovakia and South Africa. Four ‘generic price link’ countries (Belgium, Bulgaria, Finland and Turkey) informed us that they do not apply a price-link policy for biosimilars. A further 10 European countries and Canada apply a generic price link but did not report whether they also use this policy for pricing biosimilar medicines.
In most of the countries that apply a generic and biosimilar price link, the price difference between the originator medicine and the biosimilar is, sometimes considerably, lower than that between originator and generic medicine. This implies that biosimilar medicines tend to have higher prices. For instance, in the Czech Republic the first generic drug must be priced 32% below that of the originator, whereas the price of the first biosimilar must only be 15% lower than the originator. Six countries (Austria, Iceland, Italy, Kazakhstan, Latvia and South Africa) apply the same price link for generic and biosimilar medicines. However, the design of this link is heterogeneous. In Italy for example, a Decree was passed that treats generics and biosimilars in the same way in the procedure of reimbursement. Both can automatically be reimbursable and classify for the same reference group as their originator, if the price proposed by the respective marketing authorization holder is favourable to the Italian Health Service. Austria (at the time of the survey, for information on further developments see the Discussion paragraph) and Latvia, on the contrary, have defined a percentage threshold under which the first follower – either generic or biosimilar – must be priced (48% and 30%, respectively), and percentage rates of how much the prices of further ‘followers’ must be lower than of previous generics or biosimlars. In Iceland, the price link is calculated based on the maximum wholesale price allowed for generic and biosimilar medicines. Figures 1 and 2 describe price-link policies for biosimilars and generics, including price differences.
Some countries in the survey described the use of tendering to procure biosimilars. Iceland and the UK for instance have been tendering for medicines, including biosimilars, in the inpatient sector. In Denmark, all medicines (including biosimilars) for the inpatient sector are procured by a national procurement agency (AMGROS) which is owned by the five ‘health regions’ [25]. The Norwegian Drug Procurement Cooperation is responsible for purchasing medicines for public hospitals through annual tender processes. To ensure the acceptance of the awarded products, the results of the tender process and recommendations are presented by an expert group to affected stakeholders (industry, patient organizations, doctors) [26]. A similar approach is applied in Italy, however, in a decentralized manner; 20 Italian Regional Health Authorities (RHA) are responsible for planning healthcare services and allocating financial resources. All RHAs have established an organization for purchasing goods and services and two of them (Emilia-Romagna and Tuscany) additionally appointed a separate authority for procuring medicines. Various tenders for off-patent biologicals are conducted at regional levels [27]. In Spain, a pilot project of centralized procurement was reported to have taken place for the glycoprotein hormone and anaemia treatment erythropoietin (EPO).
Tendering in the outpatient sector is particularly used to procure for ‘public functions’, e.g. vaccines, centralized procurement in emergency situations such as pandemics [28]. Cyprus and Malta, both countries in which pharmaceutical services are provided separately by a public and a private sector, procure medicines (including biosimilars) for the public sector through tendering [29–31]. Some European countries have introduced tenders or tender-like systems in the outpatient sector; public payers launch tender calls for medicines that have generic (same active ingredient) or therapeutic alternatives. The lowest bidder will be either warded the contract to supply the whole market or will be granted a preferential position on the reimbursement list, e.g. through higher coverage. Such a policy is applied in The Netherlands, where through the ‘preferential pricing policy’ health insurers tender for the lowest-priced off-patent medicine [28, 32, 33]. However, biosimilar medicines were only recently included in the tenders, and only by a limited number of insurers [25]. A tender-like procedure is also applied in the Danish off-patent outpatient market, which also includes biosimilar medicines. In their system, pharmaceutical companies submit bi-monthly price bids and the lowest-priced medicines are selected for full reimbursement within a two-week period [25, 33–36].
Demand-side measures to encourage biosimilar uptake
Prescribing by INN is a measure enforced by doctors that supports the uptake of generics as well as biosimilars. INN prescribing is in place in 35 European countries, Canada and South Africa, and is mandatory in 14 of the surveyed countries. It is only in Austria, Denmark, Serbia and Sweden that prescription by INN is not permitted, see Figure 3.
Another key demand-side measure to enhance the uptake of off-patent medicines is to allow community pharmacists to substitute the originator medicine with an off-patent medicine. Generics substitution is a commonly used practice. It is applied in 37 of the 42 countries (it is not permitted in Austria, Bulgaria, Denmark, Luxembourg and Serbia), and mandatory in 15 countries. In contrast, substitution of biosimilar medicines is only in place in some, mainly Central and Eastern European countries: Belarus, Cyprus, Czech Republic, Estonia, France, Iceland, Israel, Kazakhstan, Latvia, Malta, The Netherlands, Poland, Russia, Slovakia, Slovenia and Turkey. In some countries, e.g. Latvia, the substitution of an originator medicine by a biosimilar has not been explicitly implemented, but INN prescribing is obligatory and the medicine of the lowest price must be dispensed in the pharmacy, e.g. Latvia. There is usually no specific legal basis for biosimilar substitution; no law explicitly prohibited biosimilar substitution. In France, biosimilar substitution was introduced by the Social Insurance Law at the beginning of 2014 but is not yet in practice. Other countries reported opposition to biosimilar substitution by some stakeholders. In the Czech Republic for example, the Chamber of Pharmacists recommended against biosimilar substitution.
Pricing and reimbursement policies for biosimilar medicines, as for generics, are embedded in the overall pricing and reimbursement framework. Policies for biosimilar medicines might be expected to be similar to those for generics, yet our survey showed that this is the case for only some policies and varies by country.
With regard to pricing of generic and biosimilar medicines, there are, in principle, two different approaches: to allow free pricing for off-patent medicines, and to allow for competition. The incentive for pharmaceutical manufacturers to offer low prices is to achieve higher market shares, since the lowest-priced medicines are likely to obtain more public funding and/or be recommended to pharmacists, doctors and patients. Reimbursement strategies, such as a reference price system or tendering for off-patent medicines, likely support competition.
An alternative policy is price regulation, typically in the form of a price link, whereby the price of a generic or biosimilar medicine is determined in relation to the originator price. This pricing policy appears to be commonly applied for generic medicines, even in combination with external price referencing (international price comparison) in several countries including Belgium, Hungary, Poland and Spain. In some countries, a price-link policy is applied for biosimilars as well. All countries that apply the price-link policy for biosimilar medicines do the same for generics. The survey showed, however, that several countries with a price-link policy for generics do not have one for biosimilar medicines. While four generic price link countries explicitly advised that they do not apply a price link for biosimilars, other countries with a generic price-link policy did not respond to the question about the use of price linkage for biosimilars. This suggests that legislation on this issue has not yet been decided, likely due to the novelty of the topic.
In 2016, a few countries, e.g. Austria, South Africa, did not apply specific pricing regulation for biosimilar medicines. They used the same procedures for all off-patent medicines, whether or not these medicines were generics or biosimilars (in Austria, for instance, legislation valid at the time of the survey referred only to ‘follower products’). Furthermore, the required price difference between the originator and follower medicine did not distinguish between biosimilars and generics in these countries. In most countries, however, the price difference was lower for the originator-biosimilar pair compared to the originator-generic pair. This indicates that competent authorities in these countries grant comparatively higher prices to biosimilar medicines.
Few large-scale price comparisons include biosimilar medicines, and therefore information about the impact of the two approaches for pricing biosimilars (price link versus competition) is not available. For generics, an illustrative study of selected active ingredients [37] showed that countries that base their pricing policy for generic medicines on competition tend to have a larger price difference between the originator and the generic medicine, and generics prices are often (but not consistently) lower [7, 38–41].
Overall, tendering appears to be an effective instrument to generate savings for public payers. Norway for example, reported huge discounts for biosimilar infliximab (minus 72% in 2015) [26]. Research into biosimilar tenders by regions of Italy revealed that for 191 analyzed lots referring to three off-patent biologicals (somatropin, epoetin and filgrastim) mentioned in 24 tenders performed between 2008 and 2012, the price of filgrastim and epoetin dropped considerably, whereas the price of somatropin remained steady. Somatropin had the lowest mean number of competitors (1.16), while filgrastim had the highest (2.75) [16]. Both Norway and Italy applied tenders targeted at biological and biosimilar medicines in the hospital sector. This is in line with the results of the EBE study on pricing and reimbursement policies for biological medicines, which showed that, while biological medicines are subject to tenders in several European countries, these are hospital tenders in the majority of cases [18]. Despite evidence of its effectiveness, tendering has rarely been applied for biosimilar medicines in the outpatient sector. Dutch health insurers are experienced in tendering for off-patent medicines but have traditionally refrained from including biosimilars in their tenders [42, 43]. Only recently have some Dutch insurers started launching tenders for biosimilars [25]. According to Dutch respondents, this is to be seen in the light of physician reluctance towards biosimilar medicines; while switching from the originator to a biosimilar medicine is allowed and would be appreciated by the competent authorities, it is not yet common practice.
However, pricing is only one aspect of encouraging generics and biosimilars use. Policies are also required that ensure the use of lower-priced medicines instead of higher-priced originator medicines. It is of key importance that patients trust generics and biosimilars; otherwise, they will insist on receiving the originator medicines even if they must pay more. Health professionals such as doctors and pharmacists play a key role in this respect, as their contributions in some countries such as Germany and Norway have shown [15, 44]. Health professionals must themselves understand the value of generics and biosimilars in order to communicate it to the patients. Thus, education and possibly incentives for health professionals are needed [45].
Much debate has centred around interchangeability and switching from originator medicines to biosimilars. Recent studies have been launched to prove the safety of switches [46], such as the NOR-SWITCH study, whose preliminary results suggest that a switch from originator infliximab to biosimilar infliximab is safe [47].
The Australian government announced in early 2015 that biosimilar medicines can be substituted by pharmacists based on the clinical recommendations of the Pharmaceutical Benefits Advisory Committee. The same rules that apply to generics also hold for biosimilars, and pharmacists are permitted to substitute any biosimilar medicine for an originator product, in the absence of clinical evidence to the contrary [48]. In Europe, however, biosimilar substitution has not been widely implemented, despite advanced generic medicines substitution. Countries that allow biosimilar substitution (or, at least, do not explicitly prohibit it) have been confronted with opposition by pharmacists and doctors.
It is important to note the limitations to this survey study. It concerns a new area for which data are scarce, and knowledge is limited. Since in many cases the literature does not provide conclusive information, we used primary data from members of the PPRI network, who are experts in the field of pharmaceutical pricing and reimbursement. However, we experienced a lower response rate to some of the specific questions related to biosimilar medicines. This could be a reflection of the novelty of the area for which specific policies are yet to be defined. Due to the novelty of the topic, terminology was not fully clear to all respondents, e.g. the distinction between switching by doctors and substitution by pharmacists. We addressed these challenges by providing definitions, arranging a debate of preliminary findings during a face-to-face meeting and organizing a second round of the survey based on a slightly revised questionnaire. We also aimed to validate responses using the literature, although this was not possible in several cases due to a lack of information or contradictions between sources, e.g. information on biosimilar substitution. This makes it difficult for us to discuss our findings in the light of existing literature. Further, our findings are not comprehensive. For instance, we did not survey the ‘switch climate’ or regulations for switches in different countries, as we felt that existing research had already covered these areas. The findings refer to the situation at the time of the survey (Spring 2016); in the meantime, changes in legislation might have occurred (as with the price-link policy in Austria, for instance, when in April 2017 different percentage rates for the price difference of the originator-biosimilar pair and of the originator-generic pair were introduced). Finally, this research is descriptive, and does not assess the possible impacts of policies on biosimilar prices or uptake.
Despite these limitations, we believe that our study provides interesting and updated results. Apart from the EBE study published in the same journal [18], this is the sole recent study that considers biosimilar and generic medicines policies across a large number of countries, including several non-European nations. The study also provides value by surveying both generic and biosimilar medicines, allowing a comparison between these two groups of medicines. Such a comparison has not been provided by past studies, including the above-mentioned [18], which only focused on biological and biosimilar medicines. The broad focus of our study (generic and biosimilar medicines policies) offers novel information. Due to the novelty of the topic, it is difficult to compare our findings with those of other research.
This study provides information about pricing policies and demand-side measures to enhance the uptake of biosimilar medicines for over 40 countries and compares them to practices applied for generics. Some aspects surveyed here have not been previously discussed in the literature.
Overall, the study shows that European countries have made good use of available policies for pricing generics and enhancing their uptake. However, with regard to biosimilar medicines, policymakers in several countries appear to be struggling to identify the most appropriate approach. Indeed, in many countries, pricing and usage-enhancing policies for biosimilars have not yet been defined. Policymakers do not always apply instruments that have been successfully implemented for generics to biosimilar medicines. The reluctance to do so might result from opposition to biosimilar medicines expressed by some stakeholder groups, such as physicians.
There is a need for further research to investigate the possible impacts of biosimilar medicines policies on prices, uptake and expenditure. Given the ongoing development of policies for biosimilar medicines, such studies need to be designed with a long-term perspective. Descriptive surveys, such as this manuscript, on poli cies and practices will help to inform such impact assessments.
Biosimilar medicines have the potential to increase patient access to medicines. Their prices are lower than those of originator medicines, which help to make biological medicines more affordable. Biosimilar medicines contribute to reduced pharmaceutical expenditure and thus free financial resources, ultimately allowing a greater number of patients to be treated. Policymakers are called upon to introduce policies for the pricing, funding and promotion of biosimilar medicines in order to take advantage of these benefits. However, successful implementation of pharmaceutical policies related to biosimilar medicines, as described in this article, requires patients’ understanding and acceptance. This research aims to contribute to patient knowledge in this area.
Country abbreviations
AL: Albania; AT: Austria; BE, Belgium; BG: Bulgaria; BY: Belarus; CA: Canada; CH: Switzerland; CY: Cyprus; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; EL: Greece; ES: Spain; FI: Finland; FR: France; HR: Croatia; HU: Hungary; IE: Ireland; IL: Israel; IS: Iceland; IT: Italy; KG: Kyrgyzstan; KZ: Kazakhstan; LT: Lithuania; LU: Luxembourg; LV: Latvia; MT: Malta; NL: The Netherlands; NO: Norway; PL: Poland; PT: Portugal; RS: Republic of Serbia; RU: Russia; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; TR: Turkey; UA: Ukraine; UK: United Kingdom; ZA: South Africa.
Contributors: The authors thank their colleagues Ms Nina Zimmermann and Ms Margit Gombocz in the Pharmaceutical Pricing and Reimbursement Information (PPRI) Secretariat for their support in the data collection of the survey. Furthermore, we gratefully acknowledge the answers to the survey provided by PPRI network members.
Funders: No funding was received for writing this manuscript. The Austrian Federal Ministry of Health and Women’s Affairs financially supports the Pharmaceutical Pricing and Reimbursement Information (PPRI) Secretariat.
Prior presentations: Preliminary results related to one aspect of the presented research (pricing policies for biosimilar medicines) were presented at the ISPOR 19th Annual European Congress in Vienna, Austria, 31 October – 2 November 2016.
Funding source: The authors gratefully acknowledge the support of the Austrian Federal Ministry of Health and Women’s Affairs to the Austrian Public Health Institute for running the PPRI (Pharma ceutical Pricing and Reimbursement Information) Secretariat that is managed by the authors and colleagues. The members of the PPRI network (competent authorities for pharmaceutical pricing and reimbursement) supported this research by providing data and information for this manuscript. No funding for writing this manuscript was received.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Sabine Vogler, PhD; Peter Schneider, MA
WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Pharmacoeconomics Department, Gesundheit Österreich GmbH (GÖG/Austrian Public Health Institute), 6 Stubenring, AT-1010 Vienna, Austria.
References
1. European Commission. Carone G, Schwierz C, Xavier A. Cost-containment policies in public pharmaceutical spending in the EU. Economics and Financial Afairs, 2012 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://ec.europa.eu/economy_finance/publications/economic_paper/2012/pdf/ecp_461_en.pdf
2. World Health Organization. WHO Guideline on country pharmaceutical pricing policies. Geneva: World Health Organization, 2013 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://www.who.int/medicines/publications/pharm_guide_country_price_policy/en/
3. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilars Initiative (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
4. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
5. Simoens S, De Coster S. Sustaining generic medicines markets in Europe. J Generic Med. 2006;3(4):257-68.
6. Simoens S. Sustainable provision of generic medicines in Europe. Leuven, The Netherlands: KU Leuven 2013.
7. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilars Initiative (GaBI Journal). 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
8. Vogler S, Zimmermann N. How do regional sickness funds encourage more rational use of medicines, including the increase of generic uptake? A case study from Austria. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(2):65-75. doi:10.5639/gabij.2013.0202.027
9. Dylst P, Vulto A, Simoens S. The impact of reference-pricing systems in Europe: a literature review and case studies. Expert Rev Pharmacoecon Outcomes Res. 2011;11(6):729-37.
10. Godman B, Wettermark B, Bishop I, Burkhardt T, Fürst J, Garuoliene K. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
11. World Health Organization. Cameron A, Laing R. Cost savings of switching private sector consumption from originator brand medicines to generic equivalents. World Health Report, Background Paper, 35. Geneva 2010 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://www.who.int/healthsystems/topics/financing/healthreport/35MedicineCostSavings.pdf
12. World Health Organization. Regional Office for Europe. Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research. Copenhagen, 2015 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://www.euro.who.int/en/healthtopics/Health-systems/health-technologies-and-medicines/publications/2015/access-to-new-medicines-in-europe-technical-review-of-policy-initiativesand-opportunities-for-collaboration-and-research-2015
13. Derbyshire M. Patent expiry dates for best-selling biologicals. Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(4):178-9. doi:10.5639/gabij.2015.0404.040
14. Haustein R, de Millas C, Höer A, Häussler B. Saving money in the European healthcare systems with biosimilars. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):120-6. doi:10.5639/gabij.2012.0103-4.036
15. Mack A. Norway, biosimilars in different funding systems. What works? Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(2):90-2. doi:10.5639/gabij.2015.0402.018
16. Curto S, Ghislandi S, van de Vooren K, Duranti S, Garattini L. Regional tenders on biosimilars in Italy: an empirical analysis of awarded prices. Health Policy. 2014;116(2-3):182-7.
17. European Commission. What you need to know about biosimilar medicinal products. Consensus Information Document [homepage on the Internet]. [cited 2017 May 26]. Available from: http://www.medicinesforeurope.com/wp-content/uploads/2016/03/biosimilars_report_en.pdf
18. Acha V, Allin P, Bergunde S, Bisordi F, Roediger A. What pricing and reimbursement policies to use for off-patent biologicals? – Results from the EBE 2014 biological medicines policy survey. Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(1):17-24. doi:10.5639/gabij.2015.0401.006
19. Mestre-Ferrandiz J, Towse A, Berdud M. Biosimilars: how can payers get longterm savings? Pharmacoeconomics. 2016;34(6):609-16.
20. Renwick MJ, Smolina K, Gladstone EJ, Weymann D, Morgan SG. Postmarket policy considerations for biosimilar oncology drugs. Lancet Oncol. 2016;17(1):e31-8.
21. Mendoza C, Ionescu D, Radière G, Rèmuzat C, Young K, Toumi M. Biosimilar substitution policies: an overview. Value Health. 2015;18(7):A525.
22. Drozd M, Szkultecka-D bek M, Baran-Lewandowska I. Biosimilar drugs–automatic substitution regulations review. Polish ISPOR chapter’s Therapeutic Programs and Pharmaceutical Care (TPPC) task force report. J Health Policy Outcomes Res. 2014;1:52-7.
23. Vogler S, Leopold C, Zimmermann N, Habl C, de Joncheere K. The Pharmaceutical Pricing and Reimbursement Information (PPRI) initiative–experiences from engaging with pharmaceutical policy makers. Health Policy Technol. 2014;3(2):139-48.
24. World Health Organization. List of participating Member States [home page on the Internet]. [cited 2017 May 26]. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/european-immunization-week/european-immunization-week-20052015/european-immunization-week-2012/list-of-participating-member-states)
25. Gombocz M, Vogler S, Zimmermann N. Ausschreibungen für Arzneimittel: Erfahrungen aus anderen Ländern und Umsetzungsstrategien für Österreich. 2016.
26. GaBI Online – Generics and Biosimilars Initiative. Huge discount on biosimilar infliximab in Norway [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Biosimilars/General/Huge-discount-on-biosimilar-infliximab-in-Norway
27. Curto A, Van de Vooren K, Garattini L, Lo Muto R, Duranti S. Regional tenders on biosimilars in Italy: potentially competitive? Generics and Biosimilars Initiative (GaBI Journal). 2013;2(3):123-7. doi:10.5639/gabij.2013.0203.036
28. World Health Organization. Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies. Leopold C, Habl C, Vogler S. Tendering of pharmaceuticals in EU Member States and EEA countries. Results from the country survey. Vienna: ÖBIG Forschungs- und Planungsgesellschaft mbH, 2008 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/BooksReports/Final_Report_Tendering_June_08.pdf
29. Petrou P, Talias MA. Tendering for pharmaceuticals as a reimbursement tool in the Cyprus public Health Sector. Health Policy Technol. 2014;3(3):167-75.
30. Wouters OJ, Kanavos PG. Transitioning to a national health system in Cyprus: a stakeholder analysis of pharmaceutical policy reform. Bull World Health Organ. 2015;93(9):606-13.
31. Vogler S, Habl C, Leopold C, Rosian-Schikuta I, de Joncheere K. PPRI Report. Vienna: (PPRI), 2008. Available from: https://ppri.goeg.at/Downloads/Publications/PPRI_InterimTechnicalReport.pdf
32. Habl C, Vogler S, Leopold C, Schmickl B, Fröschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich; Wien: ÖBIG Forschungs- und Planungsgesellschaft mbH; 2008.
33. Dylst P, Vulto A, Simoens S. Tendering for outpatient prescription pharmaceuticals: what can be learned from current practices in Europe? Health Policy. 2011;101(2):146-52.
34. Thomsen E, Er S, Schüder P. PHIS Pharma Profile Denmark. Vienna: Pharmaceutical Health Information System (PHIS), 2011.
35. Leopold C, Habl C, Vogler S, Rosian-Schikuta I. Steuerung des Arzneimittelverbrauchs am Beispiel Dänemark. Vienna: Gesundheit Österreich GmbH (Austrian Health Institute), 2008.
36. Ministry of Health Denmark/Danish Health and Medicines Authority. Flowchart of the pharmaceutical system in Denmark. Poster. 3rd International PPRI Conference on Pharmaceutical Pricing and Reimbursement Policies Challenges Beyond the Financial Crisis. 12–13 October 2015; Vienna, Austria.
37. Vogler S. How large are the differences between originator and generic prices? Analysis of five molecules in 16 European countries. Farmeconomia. Health Economics and Therapeutic Pathways. 2012;13(Suppl 3):29-41.
38. Simoens S. Developing competitive and sustainable Polish generic medicines market. Croat Med J. 2009;50(5):440-8.
39. Dylst P, Simoens S. Does the market share of generic medicines influence the price level?: a European analysis. Pharmacoeconomics. 2011;29(10):875-82.
40. Aalto-Setälä V. The impact of generic substitution on price competition in Finland. Eur J Health Econ. 2008;9(2):185-91.
41. Spinks J, Chen G, Donovan L. Does generic entry lower the prices paid for pharmaceuticals in Australia? A comparison before and after the introduction of the mandatory price-reduction policy. Aust Health Rev. 2013;37(5):675-81.
42. Progenerika. Kanavos P. Tender systems for outpatient pharmaceuticals in the European Union: evidence from The Netherlands and Germany. European Medicines Information Network (EMINet), 2012 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://www.progenerika.de/wp-content/uploads/2013/02/Anlage-2_Tendering-Report-EMINET-13OCT2012-FINAL.pdf
43. European Commission. Kanavos P, Seeley L, Vandoros S. Tender systems for outpatient pharmaceuticals in the European Union: evidence from The Netherlands, Germany and Belgium. European Medicines Information Network (EMINet), 2009 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://ec.europa.eu/docsroom/documents/7607?locale=en
44. Flume M. Regional management of biosimilars in Germany. Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(3):125-7. doi:10.5639/gabij.2016.0503.031
45. Towse A, Mestre-Ferrandiz J, Berdud M, Brown JD, Walson PD, Godman B, et al. Biosimilars: achieving long-term savings and competitive markets. Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(3):103-4. doi:10.5639/gabij.2016.0503.027
46. European Commission. Report of the multi-stakeholder workshop on biosimilar medicinal products. 2016 [homepage on the Internet]. [cited 2017 May 26]. Available from: http://ec.europa.eu/DocsRoom/documents/19302
47. Goll G, Olsen I, Jorgensen K, Lorentzen M, Bolstad N, Haavardsholm E, et al. Biosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from a 52-week randomized switch trial in Norway. Arthritis Rheumatol. 2016;68(Suppl 10).
48. IMS Health. Australia: substitution rules for biosimilars criticies by research based industry. IMS Pharma Pricing & Reimbursement. 2015;20(10): 306-2.
|
Author for correspondence: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Head of Pharmacoeconomics Department, Gesundheit Österreich GmbH (GÖG/Austrian Public Health Institute), 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2017 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/do-pricing-and-usage-enhancing-policies-differ-between-biosimilars-and-generics-findings-from-an-international-survey.html
|
Aim: To explore whether medicines used in hospitals in European countries are supplied as originators or generic medicines, and to investigate the procurement conditions, including the extent of discounts at which the medicines are provided. |
Submitted: 3 July 2014; Revised: 29 November 2014; Accepted: 4 December 2014; Published online first: 17 December 2014
In recent years, policymakers in European countries have increased strategies to improve the uptake of generic medicines. For instance, INN prescribing (i.e. prescribing medicines by active ingredient rather than brand name), generics substitution (i.e. the practice of substituting a brand name medicine with a generic equivalent), and/or a reference price system (i.e. identical or similar medicines are clustered to a reference group, and the public payer defines the maximum amount – reference price – which is used as the basis for reimbursement for all medicines in the group), have been implemented in most countries of the European Union (EU) [1–8]. These policies to enhance the prescribing of generics versus on-patent medicines have, to a lesser or greater degree, been supplemented by further measures, typically in the outpatient sector, such as prescription monitoring and budgets, information campaigns to the public and, somewhat less frequently, financial incentives for pharmacists and patients [9–14].
This is done to ensure the provision of high-quality medicines at a lower financial burden for the payer, which is either the patient or the third party payer (social health insurance institutions or National Health Service). In many European countries, the latter covers, at least partially, the cost of medicines [7]. Generics are procured at, in some cases, considerably lower prices than originator medicines and thus contribute to savings for the payers, as seen in several countries, e.g. Sweden and Scotland [12, 15–20].
Pharmaceutical policy measures have usually focused on the outpatient sector. Pharmaceutical expenditure in hospitals has been fairly constant over the years (usually 5−10% of a nation’s medicines budget), so it has not been a priority of policymakers in European countries [21]. Knowledge of pharmaceutical policies, including procurement and funding strategies, in the hospital sector in Europe has therefore been limited. While clinical issues have been covered by a large body of literature, policy-related research has been scant [22]. However, in recent years, this has been changing because of an increasing awareness of the need to learn about hospital-related pharmaceutical policies [23] and to improve the management of pharmacotherapy at the interface of the inpatient and outpatient sectors. Several countries have launched initiatives in this field [24].
This information gap is partly related to dual organization and funding of the pharmaceutical systems in the European countries. Medicines prescribed and supplied in outpatient care are funded by the third-party payer, usually the state, while the remainder has to be co-paid by the patient. The third party payer decides, based on pharmacological, therapeutic and health economic considerations, which medicines used in outpatient care are reimbursed [7]. In the inpatient sector, except for special funding models for high-cost medicines, medicines are financed out of hospital budgets, which are funded by the hospital owners, which might be the state, regions, municipalities, religious orders, or some pooled funding from taxes and social health insurance contributions, depending on the country’s organization of healthcare services [25].
The outpatient and inpatient pharmaceutical systems tend to be seen as two distinct sectors within a country, hence the increasing focus on improving the interface management of pharmacotherapy. However, it is increasingly recognized that medication started during the hospital stay can impact the future medicines prescribed after a patient has been discharged [26–33]. It has been suggested that it might be better to supply, at favourable conditions, hospitals with off-patent medicines in order to ensure the initial prescribing with these medicines. However, as far as the authors know, no study has ever looked at the availability of originators and generic medicines at the level of individual hospitals in European countries. This is increasingly essential as more standard treatments lose their patents [34, 35].
Against this backdrop, this study sets out to explore whether medicines used in hospitals in European countries are supplied as on-patent or generic medicines. Furthermore, we aim to investigate the procurement conditions, including the extent of discounts at which the medicines are provided.
The analysis for this manuscript draws from data collected during the European Commission co-funded PHIS (Pharmaceutical Health Information System) project, which aimed to survey medicine management in hospitals in European countries and to collect prices of medicines used in hospitals (particularly on-patent medicines) [36, 37]. The methodology of this study was influenced by overall methodological decisions taken earlier in that project. For instance, the data collection was done for a larger basket of medicines, predominantly on-patent oncology medicines without generic alternatives.
Selection of medicines
Out of a basket of medicines whose data we had surveyed, we selected those four molecules for which a ‘generic’ version was on the market. These were:
Table 1 provides the list of these four active ingredients indicating the ATC (Anatomical Therapeutic Chemical) code and the key therapeutic indication.
We included cardiovascular medicines because they account for high volumes in the outpatient sector, and the initial treatment in hospitals typically impacts further outpatient use [38].
At the time of the survey, the patent for clopidogrel had expired in some European countries, and not in others. In order to expand the study by another medicine not for cardiovascular treatment, we also included this blood product.
Selection of countries
We defined the following selection criteria for the countries, to ensure: 1) a geographic balance; 2) a balance between ‘old’ and ‘new’ EU Member States (acceded to the EU before and after May 2004) as well as European Economic Area (EEA)/European Free Trade Association (EFTA) countries; 3) a balance between countries with a social health insurance system and those with a general taxation-based system (national health service); 4) a balance between countries with a decentralized and a centralized procurement policy for medicines used in hospitals; and 5) a balance of countries of different economic situations. This is in line with cross-country comparisons available in the current literature [39].
Countries selected were Austria, The Netherlands, Norway, Portugal and Slovakia. We could not reach a balance in all cases, but we had countries from different geographic parts of Europe (criterion 1), at least one new EU Member State (Slovakia) and an EEA/EFTA country (Norway) (criterion 2), countries in different economic situations (criterion 5), and we had a balance regarding countries with a social health insurance system (Austria, The Netherlands, Slovakia) and those with a national health service (Portugal, Norway) (criterion 3). Centralized tendering for medicines in hospitals as a key procurement policy was organized in one country (Norway), and in two further countries it was done as a first step (Portugal) or for specific medicines (high-cost medicines; Slovakia) (criterion 4).
The selected countries had a variety of policies to enhance the prescribing and use of on-patent medicines versus generics. Table 2 provides an overview of country characteristics including their generic drug policies.
During the selection of the countries, country representatives (typically from pharmaceutical pricing and reimbursement authorities and national hospital pharmacy associations) involved in the PHIS (Pharmaceutical Health Information System) project as collaboration partners were addressed, and their support for the survey was sought. Thus, the willingness of the country representatives in our network was effectively an additional practical criterion for selection, and partially explains the selection of the countries, e.g. we did not manage to include a large country.
Survey instrument
A questionnaire was developed by the management team of the PHIS project, i.e. the authors and colleagues at their institutions. The draft methodology papers, including the questionnaire, were circulated with the PHIS Advisory Board (European Commission, Executive Agency for Health and Consumers, Eurostat, OECD, WHO Europe and WHO Headquarters) and the PHIS network members and then revised following their feedback. The methodology was piloted in two hospitals in Portugal and in one hospital in Austria, and adjustments to the questionnaire were based on the lessons learned from the pilot.
The questionnaire consisted of two parts: 1) a price survey form; and 2) a general questionnaire. The price survey form listed the selected molecules and asked for information about their availability, prices and procurement conditions in the hospitals. Information about the general availability in the country (marketing authorization) and price data for the outpatient sector (ex-factory prices for Austria, Portugal, Slovakia and pharmacy purchasing prices for The Netherlands and Norway) as of 30 September 2009 were already pre-filled with data provided by the Pharma Price Information (PPI) service of Gesundheit Österreich GmbH (Austrian Health Institute) [68]. Hospital pharmacists in the participating hospitals were asked to provide data as of 30 September 2009 on the availability, actual (real) prices at which medicines were supplied and procurement conditions, e.g. tendering processes versus direct negotiations, discounts, cost-free medicines, from the internal hospital databases. The general questionnaire contained questions about the medicines management in the surveyed hospitals.
Selection of the hospitals and data collection
The national network representatives were the ones identifying and approaching hospitals to explore their willingness to participate in the survey.
In Austria, The Netherlands, Norway and Portugal, we surveyed the data during study visits to the hospitals. Teams of at least two people, usually a researcher and a country’s representative involved in the PHIS network (from a competent authority for pharmaceutical pricing and reimbursement and/or a hospital pharmacy association), met with the hospital pharmacists and collected on site the information about the availability and procurement conditions (part 1 and 2 of the survey instrument). Only hospitals that had agreed in advance to participate in the survey were visited. Since none of the hospital pharmacists withdrew their cooperation during the study visit, the response rate was 100% in these countries.
In Slovakia, we had a mixed approach. We made study visits to three hospitals. In addition, we presented the project to hospital pharmacists during the general assembly of their national association and asked for their support by responding in writing to the price survey form and the questionnaire. Eight hospitals in Slovakia returned the filled price survey form and the questionnaire. This explains the considerably higher participation rate of hospitals in Slovakia compared with other countries.
We performed the study visits in the five countries and received the written questionnaires from Slovak hospitals between September 2009 and March 2010. On average, the study visits took about three hours per hospital1.
The survey results include data from five hospitals in Austria, three hospitals in The Netherlands, four hospitals in Portugal, two hospitals in Norway and eleven hospitals in Slovakia. We focused on general hospitals and on hospitals in public ownership. Most of the hospitals willing to participate were large hospitals, i.e. more than 500 acute care beds; or medium-sized hospitals, i.e. between 400 and 500 acute care beds. Table 3 provides an overview of the hospitals in the survey in relation to the total in the selected countries.
Data analysis
Data for all presentations (a presentation is defined as a medicine in a specific pharmaceutical form, dosage and pack size) of the four selected molecules supplied to the hospitals were collected. For the purpose of the analysis, we defined a ‘common presentation’, for which we performed the comparison of availability and procurement conditions. Findings about further presentations are also presented, see Table 4.
Terminology
This paper uses the terminology as defined in the glossary on pharmaceutical terms developed by the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies [69]. Availability is defined as follows: The product is (physically) reachable for the patient, e.g. through the most accessible/appropriate healthcare supplier(s) at all times in adequate amounts and in the appropriate dosage forms, with assured quality and adequate information – so that patients have access to the medicine’. A discount is defined as ‘a price reduction granted to specified purchasers under specific conditions prior to purchase’, whereas a rebate is ‘a payment made to the purchaser after the transaction has occurred’. Country-specific terms for discounts and rebates, e.g. ‘rappel’ in Portugal, and procurement, e.g. ‘market surveillance’ in Slovakia, will be explained in the results section (see Table 4).
Figure 1 provides an overview of the availability of the selected medicines as originator or generic versions in the hospitals of the survey. Table 4 presents further information related to the procurement conditions, including the extent and types of discounts provided. Different procurement conditions surveyed were: cost-free provision, central tendering, market surveillance and individual purchase by the single hospital.
Variations were found between the countries and molecules: Hospitals in Norway, Slovakia and also Portugal and The Netherlands had the generics version more frequently available than did hospitals in Austria. Generic clopidogrel was only available in Slovakia, where the patent had already expired. Except for Norway, originator atorvastatin tended to be supplied to hospitals more frequently than the generic version, whereas simvastatin had the highest generic availability among the surveyed cardiovascular medicines across all countries. Except for Austria, amlodipine was preferably supplied as generic versions.
With the exception of one medicine in one hospital in Portugal, all surveyed hospitals had at least one presentation of the selected medicines. Hospitals in Austria, The Netherlands and Portugal always had exactly one presentation of the active ingredients, either originator or generic versions. In a few cases hospitals in Norway and Slovakia had both originator and the generic versions of the same presentation of a cardiovascular medicine. There was one hospital in Slovakia, which already had the generic version of clopidogrel, in addition to the originator.
Norway is the only country in which the surveyed medicines were exclusively centrally tendered, and the tendered presentations, independently whether they were originators or generics, were granted comparably high discounts. In Portugal, medicines used in hospitals were centrally tendered in a first step, during which an official tendering price to be valid for some years was set, and in a second step hospitals individually purchased the medicines they needed and could sometimes negotiate lower prices. In the other countries, hospitals individually procured the medicines from their suppliers, typically manufacturers and in very few cases wholesalers. For specific medicines, some hospitals in Slovakia used an instrument called ‘market surveillance’; i.e. a limited tendering process in which bids from three suppliers were requested and evaluated.
Discounts of 100% were observed in Austria (in the case of all three cardiovascular medicines in all hospitals) and in Slovakia (in very few cases) regardless of whether the originator or a generic version was supplied. A discount of 100% means that the medicines were provided to the hospital ‘cost-free’. Discounts in Portugal were difficult to assess at product level since they were granted in the form of a so-called ‘rappel’ during the purchase process of the hospital. Rappels are ex-post rebates, usually implemented at the end of the calendar year, with a bundling element, since they were granted to hospitals for a specific sales volume of all medicines of a supplier during a year.
The study looked at the availability of a small sample of medicines in hospitals in medium-sized European countries, to learn whether these medicines were available and in which form. We found that, with one exception, all surveyed medicines were available in all 25 surveyed hospitals. One hospital in Portugal did not have simvastatin; we were informed that the hospital had decided against procuring it since other medicines (atorvastatin) were considered sufficient as therapeutic choice.
The findings of our study, though exploratory due to the limited number of medicines and hospitals surveyed, highlight differences in availability between the outpatient and the hospital sectors. The selected medicines were marketed and were provided in community pharmacy in several different formats (different dosages and pack sizes) in the five countries (information provided by the PPI service [68]), but the hospitals usually had very few formats of a medicine, in most cases often exactly one format. This confirms that dispensing practices in hospitals are different than those in community pharmacies. In hospitals, single-dose packing is applied, whereas in the outpatient sector the full pack is dispensed to the patient who should then take the medication according to the instructions. In hospitals, therefore, medicines of a pack (large packs are purchased) are generally used for several patients.
Given the focused availability in hospitals, pharmaceutical companies are incentivized to be the sole supplier of a molecule to the hospitals. Even if the sales volume per product might be limited in the single hospitals, supplies for inpatients can be considered as strategically important for those medicines which will be used for long-term treatment in outpatient care after the patient’s discharge from hospital. Particularly if there are no or limited policies to enhance generics use in the outpatient sector (such as mandatory generics substitution, INN prescribing, information campaigns to the public, see Table 2 for an overview of these policies in the five countries), a change to the generic drug might be unlikely once treatment was started with an on-patent product. Reluctance by general practitioners to switch from the originator to a generic drug was even reported in Germany [70], a country with active generic drug promotion policy resulting in overall high generics uptake [71, 72]. Cardiovascular medicines, whose availability in hospitals we surveyed, are a typical example [27, 28, 38].
The stakeholders involved have conflicting objectives. Suppliers have a commercial interest to gain market shares, ideally to ensure sales volumes in the long run, whereas payers aim to keep costs down. Since several European countries have different public payers and/or different funding sources for the pharmaceutical bill in the outpatient and inpatient sectors [21, 22, 25, 63], the outpatient and inpatient payers are incentivized to shift treatments, patients, and costs from one sector to another. This is likely to impact the health outcomes of patients negatively, since such situations can irritate patients, and lead to medication errors [73–77].
Given these organizational and financial frameworks, the findings of our study are not surprising. Hospital pharmacists are under financial pressure to procure best prices within the existing pharmaceutical budgets, and they will thus purchase from those suppliers who offer the highest discounts. The purchasers’ approach is illustrated by the data that we collected on Austria. Austria is one of a few European countries in which cost-free provision of medicines to hospitals is allowed [22, 25, 40]. This procurement strategy is commonly used, at least for some medicines. In general, hospitals in Austria were not able to obtain discounts for new on-patent medicines, or to receive them as cost-free medicines [22, 25, 63, 78], but the five hospitals of the survey received the three cardiovascular medicines cost-free, two of which were originator brand-name medicines. We do not know whether generics manufacturers were in the position to offer as high discounts as the originator industry. Suppliers of originator medicines may benefit from longer-lasting business relationships with the hospitals and can adapt the marketing strategy in advance of patent expiry. Furthermore, suppliers that offer a range of medicines for different indications may be able to offer specific delivery conditions, including some kind of bundling. The cost-free provision of originator cardiovascular medicines in hospitals may considerably impact the continued use of originator medicines in outpatient care, particularly since Austria does not have INN prescribing, generics substitution or a reference price system [1]. Given the limited demand-side measures to enhance generics uptake, see Table 2, sickness funds (social health insurance institutions) as public payers for outpatient medication are required to constantly undertake information activities and prescribing monitoring. Due to dual financing, the key target groups are prescribers in the outpatient sector, and only recently sickness fund launched information activities addressing prescribers and staff in hospitals, however as a voluntary initiative, since social health insurance is not responsible for funding medicines in hospitals [10].
Stakeholders react in response to the incentives provided by the system. Interviews in Austria confirmed that hospital pharmacists were aware of the dilemma of supporting the start of a treatment with an originator medicine, but since they are responsible to their hospital administration, they will act in the best interest of the hospital.
The purchasing power of a single hospital might be questioned. Even if some hospitals might be able to obtain higher discounts, e.g. hospitals in Slovakia, overall the findings displayed rather small differences in discounts between the hospitals, and they suggested limited headroom of the individual hospitals to negotiate large price reductions. Central tendering is likely to be connected with stronger purchasing power. Norway has decided that medicines for all public hospitals are centrally procured by the public procurement agency LIS (Legemiddelinnkjøpssamarbeid, Drug Procurement Cooperation). LIS defines preferred presentations and tenders for them. Medicines not tendered by LIS, e.g. different pack sizes, dosages, can be purchased individually by the hospitals [49]. The data from our survey showed that LIS tended to define a generic as a preferred version of the surveyed cardiovascular medicines. It was reported that hospitals in Norway had individually purchased some other ‘non-preferred’ presentations of these molecules, and they were not granted any discounts. The example of Norway could be considered as good practice since it combines financial elements with awareness-raising activities targeting prescribers. LIS staff have performed extensive information activities in hospitals to inform doctors about the defined presentations, and the rationale behind for selecting them. At the same time, the practice highlights the relevance of financial incentives that, in the case of Norway, support measures to enhance generics use in hospitals. In a country such as Austria, on the other hand, generics promotion activities of hospital pharmacists (via the Drugs and Therapeutics Committee, for instance, and their generics substitution which has been performed in hospitals for years but is not permitted in the outpatient sector) [40] are less effective since a limited number of generics are available due to cost-free provision of the originators to hospitals.
We also included clopidogrel in the basket of surveyed medicines even if the patent had only expired in Slovakia at the time of the survey. We learned in the interviews that hospital pharmacists in the other countries were eagerly awaiting the patent expiry because they aimed to change to the generics version as soon as possible, and obtain larger discounts and even cost-free provision in the case of Austria. However, hospital pharmacists might not see their expectations fulfilled given the controversy regarding generic clopidogrel, which was launched as a different salt with fewer indications initially [79, 80].
Our study has several limitations. A major limitation concerns the small basket of medicines, with only three cardiovascular medicines plus clopidogrel, which is still patented in most of the countries studied. In addition, the number of hospitals varied among the countries and was low in some countries. We were not always able to obtain complete information on the procurement conditions, particularly on the discounts, since in Portugal the ‘rappel’, an ex-post bundling rebate, allowed at best estimates on the product-specific price reductions. Some limitations were related to the medicine procurement system in a country, such as the Portuguese ‘rappel’. Also, this study was a follow-up of a larger study within the PHIS project, in which we surveyed more medicines, particularly on-patent medicines without any generic alternatives. The overall setting of the EU funded PHIS project has, to a large extent, contributed to some methodological decisions. Competent authorities and hospital pharmacists, who were already members of the PHIS network, were involved as cooperation partners in the survey, and not academics. Finally, we acknowledge that the study focused on the aspect of procurement of medicines to hospitals and, though we discussed the implications of the supply of originators and generics versions for the overall healthcare system, in the light of existing generic drug policies in the outpatient sector, the issue of improving use of generics was not within the scope of this study.
Therefore, this study can only be considered as an exploratory piece of research. We recommend repeating the survey, applying the same survey design, but with a larger basket of medicines (including medicines with generics available due to recently expired patents) and including more hospitals and countries.
Notwithstanding its limitations, the study provides important new evidence. Medicines procurement policies and management in European hospitals have been overlooked by researchers and policymakers for a long time, and the investigation of pharmaceutical policies in the inpatient sector has been called for [23]. To our knowledge, the availability of originator and generic medicines in European hospitals has never been surveyed. In the EU and other high-income countries, including the US and Australia, studies on the availability of medicines have been limited to the outpatient sector, and availability not measured at the level of the single healthcare provider (pharmacy, retailer) but at the national level [81, 82]. Primary data collected on availability and prices of medicines in single healthcare units and dispensaries (e.g. hospitals, clinics, pharmacies, and retailers) have been performed based on the WHO/HAI methodology [83] in some low- and middle-income countries [84, 85] but not in high-income countries. We were able to survey additional data about the procurement conditions; particularly discounts and rebates considered as confidential information.
The study confirms the general availability of the selected medicines, and, at the same time, highlights the strategy of hospitals to be focused on one or a few presentations of a molecule. If generic alternatives are available, generics tend to be supplied to the hospitals but this is not always the case. Cardiovascular medicines, which were studied in this survey, are of relevance for both industry and public payers, since they account for high volumes due to high patient numbers and long-term use. The initial treatment in hospitals is likely to impact further medicine use in the outpatient sector and to result in a continuation of the same brand, especially if pharmaceutical policies do not encourage a switch to a generic version. The study provides a good starting point to learn about originator and generic medicines use in hospitals. The findings suggest the need to develop policies that support a more integrative healthcare system, e.g. via joint funding models for the outpatient and inpatient sectors, in order to improve medicine management at the interface of outpatient and inpatient sectors.
For patients it is important to obtain the medical treatment they require. Availability of and access to medicines is one major element. The selected medicines were found to be available in the surveyed hospitals.
Generics provide an opportunity for a more rational use of medicines and for savings to public payers. Starting treatment with generics in the hospitals would be appreciated: public payers would achieve savings, and patients would continue in outpatient care with the medication they started. The study showed that in case of the generics alternatives available these are used in some but not all hospitals. In addition, the study suggests the need for improved pharmaceutical policies at the interface of the outpatient and inpatient sectors. Limited interface management directly impacts patients in a negative way, and can contribute to confusion, irritation and even deteriorated health outcomes of the patient.
We thank the hospital pharmacists of the 25 hospitals for their willingness to participate in this study. We are very grateful that they took the time to answer our questions during the interviews and that they shared with us the data which were of confidential character in most cases. Since we assured anonymity to the hospitals concerned, we do not disclose the names of data providers. We are grateful to our (former) colleagues Claudia Habl, Christine Leopold and Simone Gritsch (Gesundheit Österreich GmbH), and Barbara Bilancikova (SUKL) for their involvement in the methodology development and survey at the time of the PHIS (Pharmaceutical Health Information System) project. We thank the PHIS Advisory Board (Jérôme Boehm, Artur Furtado, Aders Lamark Tysse, Giulia del Brenna, Christophe Roeland, Stefaan van der Spiegel of the European Commission; Anna Thuvander, Jurgita Kaminskaite of the (then) Executive Agency for Health and Consumers, Dorota Kawiorska of Eurostat, Elizabeth Docteur, Valérie Paris of OECD, Kees de Joncheere of WHO Regional Office for Europe, Richard Laing and Dele Abegunde of WHO Headquarters – institutional affiliations refer to the time of the PHIS project) as well as the PHIS network members for their feedback to draft methodology papers. In particular, we greatly appreciate the support of the PHIS network members of selected countries in identifying and addressing hospitals for cooperation.
Competing interests: The authors have no reported conflicts of interest. The methodology development and the survey was done within the framework of the PHIS (Pharmaceutical Health Information System) project that was commissioned by the Executive Agency for Health and Consumers (EAHC) under the call for proposals 2007 in the priority area ‘health information’ of the European Commission, Directorate-General Public Health and Consumers, and was co-funded by the Austrian Federal Ministry of Health.
As a follow-up of the PHIS project, we analysed the data of medicines with a generic version available with regard to their availability and procurement conditions, and we prepared this manuscript. No separate funding was provided for the supplementary analysis regarding the research question and the drafting of this manuscript.
Provenance and peer review: Commissioned; externally peer reviewed.
Sabine Vogler1, PhD
Nina Zimmermann1, MA
Jan Mazag2, PharmaDr
1WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG, Austrian Health Institute), 6 Stubenring, AT-1010 Vienna, Austria
2Statny Ustav pre Kontrolu Lieciv (SUKL, State Institute for Drug Control), 11 Kvetná, SL-82508, Bratislava 26, Slovakia
References
1. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilars Initiative (GaBI Journal). 2012;1(2):93-100. doi: 10.5639/gabij.2012.0102.020
2. Vogler S, Zimmermann N, Leopold C, de Joncheere K. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Review. 2011;4(2):69-79.
3. Leopold C, Vogler S, Habl C. Was macht ein erfolgreiches Referenzpreissystem aus? Erfahrungen aus internationaler Sicht [in German]/Implementing a successful reference price system – experiences from other countries. Soziale Sicherheit. 2008(11):614-23.
4. Dylst P, Vulto A, Godman B, Simoens S. Generic medicines: solutions for a sustainable drug market? Appl Health Econ Health Policy. 2013;11(5): 437-43.
5. Simoens S. A review of generic medicine pricing in Europe. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):8-12. doi:10.5639/gabij.2012.0101.004
6. Dylst P, Simoens S, Vulto A. Reference pricing systems in Europe: characteristics and consequences. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1:127-31. doi:10.5639/gabij.2012.0103-4.028
7. Vogler S, Habl C, Bogut M, Voncina L. Comparing pharmaceutical pricing and reimbursement policies in Croatia to the European Union Member States. Croat Med J. 2011;52(2):183-97.
8. Simoens S. Trends in generic prescribing and dispensing in Europe. Expert Rev Clin Pharmacol. 2008 Jul;1(4):497-503.
9. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
10. Vogler S, Zimmermann N. How do regional sickness funds encourage more rational use of medicines, including the increase of generic uptake? A case study from Austria. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(2):65-75. doi:10.5639/gabij.2013.0202.027
11. Godman B, Shrank W, Wattermark B, Andersen M, Bishop I, Gustafsson LL. Use of generics – a critical cost containment measure for all healthcare professionals in Europe? Pharmaceuticals. 2010;3(8):2470-94.
12. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Comparing policies to enhance prescribing effi ciency in Europe through increasing generic utilization: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):707-22.
13. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Policies to enhance prescribing effi ciency in Europe: fi ndings and future implications. Front Pharmacol. 2010;1:141.
14. Godman B, Wettermark B, Bishop I, Burkhardt T, Fürst J, Garuoliene K, et al. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
15. World Health Organization. Cameron A, Laing R. Cost savings of switching private sector consumption from originator brand medicines to generic equivalents. World Health Report. Background Paper, 35. Geneva 2010 [homepage on the Internet]. 2011 Sep 17 [cited 2014 Nov 15]. Available from: http://www.who.int/healthsystems/topics/financing/healthreport/35MedicineCostSavings.pdf
16. Seeley E, Kanavos P. Generic medicines from a societal perspective: savings for health care systems? Eurohealth. 2008;14(2):18-21.
17. Simoens S. International comparison of generic medicine prices. Curr Med Res Opin. 2007;23(11):2647-54.
18. Tafuri G, Creese A, Reggi V. National and international differences in the prices of branded and unbranded medicines. Journal of Generic Medicines. 2004;1(2):120-7.
19. Vogler S. How large are the differences between originator and generic prices? Analysis of five molecules in 16 European countries. Farmeconomia Health economics and therapeutic pathways. 2012;13 (Suppl 3):29-41.
20. Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):125-30.
21. Vogler S, Zimmermann N, Mazag J. Procuring medicines in hospitals: results of the European PHIS survey. Eur J Hosp Pharm Prac. 2011(2):20-1.
22. Vogler S, Zimmermann N, Habl C, Mazag J. The role of discounts and loss leaders in medicine procurement in Austrian hospitals – a primary survey of official and actual medicine prices. Cost Eff Resour Alloc. 2013;11(1):15.
23. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Report. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG, 2008. 2008 Jun 2 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/BooksReports/PPRI_Report_final.pdf
24. Björkhem-Bergman L, Andersén-Karlsson E, Laing R, Diogene E, Melien O, Jirlow M, et al. Interface management of pharmacotherapy. Joint hospital and primary care drug recommendations. Eur J Clin Pharmacol. 2013;69(1):73-8.
25. Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Pharmaceutical Health Information System (PHIS); commissioned by the European Commission and the Austran Federal Ministry of Health, 2010. 2010 Jul 16 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/BooksReports/PHIS_HospitalPharma_Report.pdf
26. Perren A, Donghi D, Marone C, Cerutti B. Economic burden of unjustified medications at hospital discharge. Swiss Med Wkly. 2009;139(29-30):430-5.
27. Gallini A, Legal R, Taboulet F. The influence of drug use in university hospitals on the pharmaceutical consumption in their surrounding communities. Br J Clin Pharmacol. 2013;75(4):1142-8.
28. Feely J, Chan R, McManus J, O’Shea B. The infl uence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics. 1999;16(2):175-81.
29. Schröder-Bernhardi D, Dietlein G. Lipid-lowering therapy: do hospitals influence the prescribing behavior of general practitioners? Int J Clin Pharmacol Ther. 2002;40(7):317-21.
30. Himmel W, Tabache M, Kochen M. What happens to long-term medication when general practice patients are referred to hospital? Eur J Clin Pharmacol. 1996;50(4):253-7.
31. Himmel W, Kochen M, Sorns U, Hummers-Pradier E. Drug changes at the interface between primary and secondary care. Int J Clin Pharmacol Ther. 2004;42(2):103-9.
32. Grimmsmann T, Schwabe U, Himmel W. The infl uence of hospitalisation on drug prescription in primary care–a large-scale follow-up study. Eur J Clin Pharmacol. 2007;63(8):783-90.
33. Bijl D, Van Sonderen E, Haaijer-Ruskamp F. Prescription changes and drug costs at the interface between primary and specialist care. Eur J Clin Pharmacol. 1998;54(4):333-6.
34. Frank RG. The ongoing regulation of generic drugs. N Engl J Med. 2007;357(20):1993-6.
35. Jack A. Balancing Big Pharma’s books. BMJ. 2008;336(7641):418-9.
36. Hoebert J, Mantel-Teuwisse A. PHIS Evaluation Report. Utrecht: Pharmaceutical Health Information System (PHIS), 2011. 2011 Apr 6 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/FurtherDocuments/PHIS evaluation report.pdf
37. Vogler S, Leopold C, Zimmermann N, Habl C, de Joncheere K. The Pharmaceutical Pricing and Reimbursement Information (PPRI) initiative–experiences from engaging with pharmaceutical policy makers. Health Policy and Technology. 2014;3(2):139-48.
38. de Vries CS, van Diepen NM, Tromp TF, de Jong-van den Berg LT. Auditing GPs’ prescribing habits: cardiovascular prescribing frequently continues medication initiated by specialists. Eur J Clin Pharmacol. 1996;50(5):349-52.
39. Cacace M, Ettelt S, Mays N, Nolte E. Assessing quality in cross-country comparisons of health systems and policies: towards a set of generic quality criteria. Health Policy. 2013;112(1-2):156-62.
40. Zimmermann N, Vogler S. PHIS Hospital Pharma Report Austria. Vienna: Pharmaceutical Health Information System (PHIS), 2009. 2009 Jun 30 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/PHIS_Hospital_Pharma_AT_Report_Final_version_090630.pdf
41. Vogler S, Zimmermann N. The potential of generics policies: more room for exploitation–PPRI Conference Report. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):146-9. doi:10.5639/gabij.2012.0103-4.030
42. Leopold C, Habl C, Vogler S, Morak S. PPRI Pharma Profi le Austria. Vienna: Pharmaceutical Pricing and Reimbursement Information (PPRI), 2008. 2008 Nov 13 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformation/Reports/Austria_PPRI_2008_Englih_Version.pdf
43. Winkelmayer WC, Asslaber M, Bucsics A, Burkhardt T, Schautzer A, Wieninger P, et al. Impact of reimbursement changes on statin use among patients with diabetes in Austria. Wien Klin Wochenschr. 2010;122(3-4):89-94.
44. Godman B, Burkhardt T, Bucsics A, Wettermark B, Wieninger P. Impact of recent reforms in Austria on utilization and expenditure of PPIs and lipidlowering drugs: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2009;9(5):475-84.
45. Storms H, Schreurs M. PHIS Hospital Pharma Report The Netherlands Vienna: Pharmaceutical Health Information System (PHIS), 2010. 2010 May 10 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/PHISNLHospital Pharma Report 2009.pdf
46. Dylst P, Vulto A, Simoens S. Tendering for outpatient prescription pharmaceuticals: what can be learned from current practices in Europe? Health Policy. 2011;101(2):146-52.
47. Dylst P, Simoens S. Generic medicine pricing policies in Europe: current status and impact. Pharmaceuticals. 2010;3(3):471-81.
48. Woerkom Mv, Piepenbrink H, Godman B, Metz Jd, Campbell S, Bennie M, et al. Ongoing measures to enhance the effi ciency of prescribing of proton pump inhibitors and statins in The Netherlands: infl uence and future implications. J Comp Eff Res. 2012;1(6):527-38.
49. Aanes T, Ognøy AH, Festøy H. PHIS Hospital Pharma Report Norway. Vienna: Pharmaceutical Health Information System (PHIS), 2009. 2009 Nov 3 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/PHISNorway Hospital Pharma Report 2009.pdf
50. Festöy H, Sveen K, Leung-Ming Y, Gjönnes L, Gregersen T. PPRI Pharma Profile Norway. Vienna: Pharmaceutical Pricing and Reimbursement Information (PPRI), 2008. 2009 Mar 9 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/ Norway_
PPRI_2008.pdf
51. Håkonsen H, Horn AM, Toverud E-L. Price control as a strategy for pharmaceutical cost containment – what has been achieved in Norway in the period 1994-2004? Health Policy. 2009;90(2-3):277-85.
52. Brekke KR, Holm TH, Straume OR. Margins and market shares: pharmacy incentives for generic substitution. European Econ Rev. 2013;61:116-31.
53. Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11(1):121-9.
54. Sakshaug S, Furu K, Karlstad Ø, Rønning M, Skurtveit S. Switching statins in Norway after new reimbursement policy: a nationwide prescription study. Br J Clin Pharmacol. 2007;64(4):476-81.
55. Caldeira S, Furtado C, Vieira I, Baptista A. PHIS Hospital Pharma Report Portugal Vienna: Pharmaceutical Health Information System (PHIS), 2010. 2011 Mar 10 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/PHIS Hospital Pharma Portugal 2010.pdf
56. Teixeira I, Vieira I. PPRI Pharma Profile. Portugal. Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information), 2008. 2008 Dec 16 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/Portugal_PPRI_2008.pdf
57. Leopold C, Mantel-Teeuwisse AK, Vogler S, Valkova S, de Joncheere K, Leufkens HG, et al. Effect of the economic recession on pharmaceutical policy and medicine sales in eight European countries. Bull World Health Organ. 2014;92(9):630-40.
58. Simoens S. The Portuguese generic medicines market: a policy analysis. Pharm Pract (Granada). 2009;7(2):74-80.
59. Leopold C, Zhang F, Mantel-Teeuwisse AK, Vogler S, Valkova S, Ross-Degnan D, et al. Impact of pharmaceutical policy interventions on utilization of antipsychotic medicines in Finland and Portugal in times of economic recession: interrupted time series analyses. Int J Equity Health. 2014;13:53.
60. Habl C, Vogler S, Leopold C, Schmickl B, Fröschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich. Wien: ÖBIG Forschungs- und Planungsgesellschaft mbH; 2008. 2008 Apr 11 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/BooksReports/EB_RPS_31_3_08.pdf
61. Quintal C, Mendes P. Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health policy. 2012;104(1):61-8.
62. Mazag J. PHIS Hospital Pharma Report Slovakia. Vienna: Pharmaceutical Health Information System (PHIS), 2009. 2009 Aug 13 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformation Reports/7SlovakiaHospital Pharma.pdf
63. Vogler S, Zimmermann N, Leopold C, Habl C, Mazag J. Discounts and rebates granted for medicines for hospital use in fi ve European countries. The Open Pharmacoeconomics and Health Economics Journal. 2013;5:1-10.
64. Mazag J, Segec A. PPRI Pharma Profi le Slovakia. Vienna: Pharmaceutical Pricing and Reimbursement Information (PPRI), 2007. 2007 May 11 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/CountryInformationReports/Slovakia_PPRI_2007.pdf
65. Kaló Z, Docteur E, Moïse P. Pharmaceutical pricing and reimbursement policies in Slovakia. Paris: OECD, 2008. [cited 2014 Nov 15]. Available from: http://dx.doi.org/10.1787/244264621247
66. Håkonsen H, Toverud E-L. A review of patient perspectives on generics substitution: what are the challenges for optimal drug use. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):28-32. doi:10.5639/gabij.2012.0101.008
67. Palagyi M, Lassanova M. Patients attitudes towards experience with use of generics in Slovakia, performance of generic substitution. Bratisl Lek Listy. 2008;109(7):324-8.
68. Gesundheit Österreich GmbH. Pharma Price Information (PPI) service. Vienna 2014. [cited 2014 Nov 15]. Available from: http://www.goeg.at/en/PPI
69. WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies. Glossary of pharmaceutical terms. Update 2013. Vienna 2013 [homepage on the Internet]. 2013 Nov 25 [cited 2014 Nov 15]. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/MethodologyTemplate/PHISGlossary_Update2013_fi nal_gesamt.pdf
70. Simmenroth-Nayda A, Hummers-Pradier E, Ledig T, Jansen R, Niebling W, Bjerre LM, et al. Prescription of generic drugs in general practice. Results of a survey of general practitioners [Article in German]. Med Klin (Munich). 2006;101(9):705-10.
71. Godman B, Schwabe U, Selke G, Wettermark B. Update of recent reforms in Germany to enhance the quality and efficiency of prescribing of proton pump inhibitors and lipid-lowering drugs. Pharmacoeconomics. 2009;27(5):435-8.
72. Garattini L, Tediosi F. A comparative analysis of generics markets in five European countries. Health Policy. 2000;51(3):149-62.
73. Gleason K, McDaniel MR, Feinglass J, Baker D, Lindquist L, Liss D, et al. Results of the Medications At Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-7.
74. Glintborg B, Andersen SE, Dalhoff K. Insuffi cient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16(1):34-9.
75. Layson-Wolf C, Morgan JA. Pharmacy continuity of care: what do community pharmacists need from an acute care hospital to improve continuity of pharmaceutical care? Disease Management and Health Outcomes. 2008;16(4): 199-203.
76. Santell JP. Reconciliation failures lead to medication errors. Jt Comm J Qual Patient Saf. 2006;32(4):225-9.
77. Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15(2):122-6.
78. Langebner T. Managing drug costs in Austria. Eur J Hosp Pharm Prac. 2007;13(5):79.
79. Baumgartel C, Godman B, Malmstrom R, et al. What lessons can be learned from the launch of generic clopidogrel? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68. doi:10.5639/gabij.2012.0102.016
80. Baumgärtel C. Generic clopidogrel–the medicines agency’s perspective. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):89-91. doi:10.5639/gabij.2012.0102.019
81. Danzon PM, Furukawa MF. Prices and availability of pharmaceuticals: evidence from nine countries. 2003;Suppl Web Exclusives:W3-521-36.
82. Danzon PM, Furukawa MF. International prices and availability of pharmaceuticals in 2005. Health Aff (Milwood). 2008;27(1):221-33.
83. World Health Organization. Measuring medicine prices, availability, affordability and price components. Geneva: WHO, 2008 [homepage on the Internet]. 2013 May 6 [cited 2014 Nov 15]. Available from: http://www.haiweb.org/medicineprices/manual/manuals/MedicinePrices.pdf
84. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240-9.
85. Mendis S, Fukino K, Cameron A, Laing R, Filipe Jr A, Khatib O, et al. The availability and affordability of selected essential medicines for chronic diseases in six low-and middle-income countries. Bull World Health Organ. 2007;85(4):279-88.
| Author for correspondence: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG, Austrian Health Institute), 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2014 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/availability-and-procurement-conditions-of-originator-and-generic-medicines-in-hospitals-an-exploratory-study-in-five-medium-sized-european-countries.html
|
Aim: To explain the components of pharmaceutical expenditure and illustrate the strengths and limitations of this indicator. In particular, we explore policies applied in European countries that affect the price and volume of medicines. |
Submitted: 10 April 2013; Revised: 7 June 2013; Accepted: 18 June 2013; Published online first: 5 July 2013
Pharmaceutical expenditure is a major indicator used in national and international statistics on pharmaceutical markets and for pharmaceutical policy analyses. Knowledge of pharmaceutical expenditure and its development is of major interest to policymakers and researchers. Baseline information on the status quo of pharmaceutical expenditure is needed to compare with other macroeconomic indicators, such as health expenditure or gross domestic product in a country or region. Pharmaceutical expenditure is also a key indicator in international comparisons. The development of pharmaceutical expenditure at national level or in international comparisons is subject to several analyses, such as assessing the effect of pharmaceutical policies or forecasting future trends [1–8].
Although pharmaceutical expenditure is a standard indicator, worldwide data are not easily accessible. The World Health Organization (WHO) World Medicines Situation Report 2011 [9] devoted an entire chapter to presenting national pharmaceutical expenditure. Data on pharmaceutical spending were extracted from national health accounts where available. Large differences in pharmaceutical expenditure among the regions of the world were confirmed: pharmaceutical expenditure per capita ranged from US$7.61 in low-income countries to US$431.6 in high-income countries in 2005 and 2006, with considerable variation between income groups in each country. On average, 24.9% of total pharmaceutical expenditure was spent on medicines, ranging from 7.7 to 67.6% [9].
The total pharmaceutical bill across the European Union reached more than Euros 190 billion in 2010 [10]. On average, pharmaceutical expenditure accounted for almost one-fifth (19%) of all health expenditure in European Union Member States in 2010, making it the third biggest spending component after in-patient (hospital) and out-patient (ambulatory) care. Pharmaceutical expenditure rose by more than 50% in real terms between 2000 and 2009 in Organisation for Economic Co-operation and Development (OECD) countries, despite negative growth rates in several countries in 2010 [10].
Other major repositories of pharmaceutical expenditure data in high-income countries include the OECD Health database [11] and the Eurostat Health database [12]. The databases are populated by respective Member States according to clear definitions and sound methodologies. In order to reduce the burden of data collection for national authorities, and to increase the use of international standards and definitions in the field of health accounting, increased cooperation between OECD, Eurostat and WHO was agreed for the collection of health expenditure data based on the System of Health Accounts methodology. This resulted in the launch of the Joint OECD–Eurostat–WHO Health Accounts Data Collection [13].
Methodological challenges of health expenditure accounting have been addressed [14–16]. Despite the wide use of pharmaceutical expenditure in science and practice, it is much less of a focus.
In this paper, we explain which components determine pharmaceutical expenditure. In particular, we explore which pharmaceutical policies able to influence price or volume are relevant to the European context. We flag up possible limitations of the pharmaceutical expenditure indicator, and highlight ways to interpret, analyse and compare pharmaceutical expenditure data.
This paper is based on a presentation held at the European Drug Utilization Research Group (EuroDURG)/International Society for Pharmacoepidemiology (ISPE) meeting ‘Better public health through pharmaco-epidemiology and quality use of medicine’ in Antwerp, Belgium, on 1 December 2011. We have since added pharmaceutical policy updates. We have also added further examples, such as price data, not previously included because of time constraints.
Data sources
We present pharmaceutical expenditure data extracted from the major health indicator databases OECD Health Data [11] and Eurostat Health Data [12]. As these data are not fully comprehensive, we have included supplemental data obtained from a primary survey of national authorities. The data-collection exercise was carried out within the framework of the Pharmaceutical Health Information System (PHIS) project [17], established on completion of the PHIS database of indicators to analyse pharmaceutical systems [18].
Updated information for pharmaceutical policy mapping in European countries was provided by the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies (Vienna, Austria), to which we are affiliated. This WHO Collaborating Centre has been regularly and systematically collecting pharmaceutical policy information in European countries [19].
We obtained price data from the Pharma Price Information (PPI) service of the Austrian Health Institute [20]. The PPI service provides information on prices of medicines set by European Union Member States, covering all price types. It was established to support, as stipulated in the Austrian General Social Insurance Law [21], the Austrian Pricing Committee located at the Austrian Federal Ministry of Health, which calculates the European Union average price. This average price is required for price setting, as Austria applies external price referencing and bases its prices on the average prices of all other European Union Member States [22].
To ensure clarity of meaning and comprehension, we explain technical terms with reference to the pharmaceutical policy glossary of the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies [23]. We also use the terms ‘out-patient care’, i.e. ambulatory care, community care, without hospital out-patient departments, and ‘in-patient’ or ‘hospital care’, as defined in this and other glossaries.
We begin by addressing price components of pharmaceutical expenditure and underlying policies influencing price, and then we present volume components and policies, and the different methodological approaches to measurement units. Different sub-markets that pharmaceutical expenditure may refer to are then analysed, and further methodological limitations are finally discussed.
Official out-patient price types
Pharmaceutical expenditure is determined by a value component and a volume component, i.e. price multiplied by volume in the (sub-)markets in question.
The usual price types for the out-patient sector are as follows:
In some cases, the latter is also referred to as ‘public price’, to indicate the price set for the public, i.e. consumers. We advise not using the term ‘public price’ because it could be misconstrued as a reference to the funding source of a ‘public payer’. The pharmacy retail price (net) can be increased by further add-ons, such as duties and taxes, which results in the pharmacy gross retail price.
These price types are shown in Figures 1 and 2, with real-life examples for four medicines from different indications (oncology, rheumatoid arthritis, diabetes, antipsychotic); generics are available for two of them. In Figure 1, prices of medicines are presented as unit prices, i.e. the price per tablet or vial, for the different price types in 16 European countries. Existing variations are highlighted among products and countries.
The different price types are attributed to a regulation in place in several high-income countries, including European countries. Medicines, or a large proportion of medicines on the market, are funded by public payers (so-called reimbursable medicines). At the manufacturer level (ex-factory prices), prices of reimbursable medicines are usually statutorily set, i.e. based on a legal act such as a law or decree, and can be negotiated between the state and the manufacturer; sometimes a combination of both procedures is applied. Different methods for setting an ex-factory price can be applied.
A frequently used approach to determine the price of new medicines is external price referencing, i.e. the practice of using the price(s) of a medicine in one or several countries to derive a benchmark or reference price for the purposes of setting or negotiating the price of the product in a given country [23]. As of 2013, 24 out of the 27 European Union Member States (all but Denmark, Sweden and UK) apply external price referencing, at least as a supportive tool, for new medicines [19, 24, 24]. At the same time, generic medicines prices are either set at a specific percentage lower than the price of the generic medicine (‘generic price link’), or countries work competitively to decrease prices, or a mixture of the two [3, 8, 26, 27].
Pharmacy purchasing and retail price types are determined through regulations stipulating maximum remuneration granted to the stakeholders involved in pharmaceutical distribution. Wholesale and pharmacy mark-ups usually apply to all medicines, but are limited to reimbursable and prescription-only medicines in some countries [18, 28]. Some European countries set a medicine price at the pharmacy level and do not have statutory wholesale mark-ups. For those countries, ex-factory prices can, at best, be calculated on the basis of an estimated average wholesale margin. The design of the distribution remuneration in the form of fixed mark-ups or regressive schemes influences pharmacy purchasing and pharmacy retail prices. Several European countries, for example, have opted for regressive wholesale and pharmacy margin schemes, which gradually decrease the mark-ups for high-cost medicines. The share of the price attributable to wholesalers, and particularly pharmacies, tends to be higher for low-price medicines in regressive margin schemes. Pharmacy remuneration may also be designed independently from the price, e.g. by providing a fee for service, such as in The Netherlands or in the UK [1, 3, 28–30].
The price of a medicine and, as a result, pharmaceutical expenditure, may be increased by duties and taxes, which are known to account for a considerable part of the medicine price in middle- and low-income countries [31–33]. In European countries, only value-added tax is applied to medicines. Most European countries apply a value-added tax rate on medicines that is lower than the standard rate, see Figure 3. When comparing pharmaceutical expenditure between countries, we strongly advise checking whether data are indicated net or gross.
‘Reimbursed price’ or ‘reimbursement price’ is important for public payers. It refers to the maximum amount covered by public payers (usually social insurance or a national health service) [23]. In European countries, the term ‘reimbursement price’ is not explicitly indicated, except for Austria, which uses the term ‘sickness fund price’. In other countries, the reimbursement price can be derived from the medicine price, usually the pharmacy retail price, by deducting the percentage of co-payments incurred by the consumers. Most European countries (all European Union Member States except Austria, Germany, Italy, The Netherlands and UK [29, 30]) ask the patient to co-pay a specific percentage of the medicine price: specific medicines, whose therapeutic (added) value was assessed to be lower compared with essential or life-saving medicines, are not fully funded by the public payers, but they are reimbursed to a specific extent. For instance, if the pharmacy retail price is Euros 100 and the reimbursement rate is 80%, then the reimbursement price amounts to Euros 80.
In this case, the patient is required to co-pay Euros 20 plus a prescription fee, if applicable, unless they are exempted from co-payment or are granted reduced co-payment.
A particular reimbursement price is called the reference price. A reference price system is a reimbursement system in which identical or similar medicines, e.g. originator medicines and generics, are clustered to a reference group, and a public payer defines the maximum price (amount) that is reimbursed for all medicines clustered to this group [23, 34]. A reference group can be clustered for the molecule (Anatomical Therapeutic Classification [ATC] Level 5), the class (ATC Level 4) or the therapeutic area (ATC Level 3) [35]. The patient is required to pay the difference between the reference price and the pharmacy retail price, plus any further co-payments, e.g. in Portugal, the percentage co-payment is also applicable to the reference price [36]. As of 2013, 20 European Union Member States have a reference price system in place [19], and Ireland decided in May 2013 to introduce it.
Price studies can be carried out for each of these price types, or a combination of them. It depends on the perspective of the analysis and which price type is selected.
Official in-patient price types
Hospitals are usually directly supplied by the pharmaceutical industry and, in some cases, by wholesalers [37, 38]. In contrast to the out-patient sector, only one official price type is applicable for the in-patient sector, and that is the ‘official hospital price’. It generally corresponds to the ex-factory price or, in the case of delivery by a wholesaler, to the wholesale price, which includes the wholesale mark-up.
Discounted prices
Discounted prices are known from the hospital sector. Large discounts and rebates are offered on specific medicines to the hospitals. In some European countries, cost-free medicines are permitted, so hospitals obtain medicines at a price of zero Euro. Discounts and rebates, including a cost-free supply, have been observed, particularly for medicines, where competitors have come onto the market, and whose use might be required for long-term treatment [37, 39]. As a result, the actual hospital price is of greater relevance than official hospital prices, as the hospital’s pharmaceutical bill is composed of what the hospital actually spends on medicines.
Discounted prices also play a role in the out-patient pharmaceutical sector. Along the supply chain, e.g. from wholesaler to pharmacies, from pharmacies to consumers, discounts can be granted, if not forbidden by law. Legal provisions may specify the maximum amount of discounts and rebates granted. In France, for example, pharmacies are permitted to obtain a maximum discount of 2.5% on reimbursable medicines from wholesalers, whereas the limit is 17% for reimbursable generics [40].
Furthermore, discounts and rebates granted to public payers are less well known, but have increasingly been gaining importance. According to a survey of European countries [41], discounts and rebates are granted to public payers by pharmaceutical companies in 25 of the 31 European countries surveyed (out-patient sector in 21 countries and in-patient sector in all 25 countries). The most common discounts and rebates are price reductions and refunds linked to sales volume, but types such as in-kind support, price-volume and risk-sharing agreements are also in place. A mix of various types of discounts and rebates is common. Risk-sharing and further managed-entry agreements that attempt to manage uncertainty are on the rise in several European countries, such as UK (patient access schemes), Italy, Poland and the Baltic States [42, 43].
Volume measurement and analysis
Different methodological approaches can be applied to measure utilization. Pharmaceutical utilization can be assessed by the number of medicine packs sold, dispensed, or actually consumed by patients. Sales data can also be indicated by ‘Standard Units’, which are defined as the smallest doses of a product, equivalent to one tablet or capsule for an oral dosage form, one teaspoon, i.e. 5 mL, for a syrup, and one ampoule or vial for an injectable product [44].
A major discipline dealing with the volume component is drug-utilization research, which WHO has defined as ‘the marketing, distribution, prescription, and use of drugs in a society, with special emphasis on the resulting medical, social and economic consequences’ [45]. From a public health perspective, a major aim of drug-utilization research is to enhance a more rational use of medicines by ensuring that patients receive ‘medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community’ [46]. Drug-utilization research describes patterns of pharmaceutical use, indicates early signals of irrational use of medicines, assesses interventions to improve the use of medicines, and benchmarks the use of the medicines in different populations [45]. This discipline has contributed greatly to statistics, and a measurement unit (Defined Daily Dose [DDD]) was developed to allow international comparisons of volume data to be made: the DDD is defined as the average maintenance dose of the medicine when used on its major indication in adults [47]. Today DDD is a standard measurement for pharmaceutical consumption; drug-utilization research would not be possible without it.
From a public payer’s perspective, the ‘items prescribed’ tend to be a focal point of analyses, as prescriptions are likely to be monitored. For this purpose, the relevant scientific measurement unit, again developed to allow comparisons to be made, is the ‘Prescribed Daily Dose’ [PDD]. It is defined as the average daily dose prescribed, as obtained from a representative sample of prescriptions [45]. The number of items prescribed might differ from the number of items actually dispensed, as not all prescriptions might be filled. It is more difficult to obtain information on the number of items dispensed [18].
Volume control policies
In European countries, volume control policies are usually targeted at medicines prescribed by physicians, and therefore prescribers are the key target group of this measure. Furthermore, demand-side measures can also be targeted at patients and pharmacists [48, 49].
In some European countries, such as the Czech Republic, Latvia, Slovakia, UK, and some regions in Spain, and Sweden, pharmaceutical budgets for prescribers are in place [19, 50–52]. Budgets can be combined with financial incentives. For example, in Ireland, doctors were permitted to plough back some of their savings into their practice under the ‘Indicative Drug Target Scheme’; this financial incentive was, however, subsequently scrapped [53]. Where budgets are exceeded, penalties may be imposed, e.g. Latvia. In the 1990s pharmaceutical budgets of countries such as France, Germany and Italy yielded the savings forecast during the first year but not in subsequent years. Sanctions were imposed but could not be executed, partly because of court proceedings. Eventually, the budgets were abolished [54].
Physician prescription patterns are routinely monitored in all European countries [1, 50] to assess volume and quality of prescribing. For instance, public payers check whether doctors prescribe less expensive generics, write prescriptions by International Nonproprietary Name (INN), or both, if that is the policy in place. Prescription monitoring has been implemented in different ways and to differing extents in Europe, and also depends on the electronic support system in place. The Danish electronic monitoring system, ‘Ordiprax’, is considered to be an example of good practice. Ordiprax allows the authorities to assess pharmaceutical consumption at the level of the prescribing doctors and at aggregated local and central levels. Doctors also have access to the Ordiprax system and can compare their prescription pattern to the average of other physicians in the region [55].
Some European countries supplement their prescription monitoring by specific agreements with physicians. For example, physicians are financially rewarded for prescribing a specific target within a therapeutic class, such as statins of generics, and less expensive medicines in France [56]; doctors in Belgium have to prescribe a minimum target of ‘non-expensive medicines’ whose exact amount depends on their medical specialisation [57, 58]. Further, prescriber information also plays a major role [59]. In France, sickness fund representatives (Délégués de l’Assurance Maladie) visit physicians to provide feedback on their prescribing activity and to inform them about campaigns [40].
The patient is another key target group, and several European countries have targeted campaigns at patients [1, 50, 59]. Although these measures might have contributed to containing public pharmaceutical expenditure, the primary aim was to encourage a more rational use of medicines. Well-known examples are the antibiotics campaign in France and Italy, and generics campaigns in France and Portugal [35, 50].
Initiatives aimed at increasing generics uptake play an important role because pharmaceutical expenditure can be reduced by using lower priced medicines, where available, instead of expensive originator medicines. Key policies to encourage generics use have been devised for different stakeholders: prescribers (INN prescribing), pharmacists (generics substitution) and patients (campaigns to raise awareness about the role and benefits of generics). At the beginning of 2013, 22 out of the 27 European Member States had generics substitution in place, and 23 allowed INN prescribing [19, 34]. In recent years, Lithuania and Slovakia, for example, decided to switch from voluntary to obligatory INN prescribing [19, 60, 61] on the basis that previous voluntary policies had not been fully enforced and tended to be less successful in achieving planned outcome than mandatory policies [1, 36]. On the other hand, high generics uptake has been achieved in the UK with voluntary INN prescribing, and a decision was made against the introduction of generics substitution planned for 2010 following a public consultation [34, 62, 63].
An econometric model has been used in 12 European countries to assess the relevance of different volume components that influence pharmaceutical expenditure. The study concluded that the following three volume control policy measures had significantly decreased pharmaceutical expenditure: an electronic prescribing system; pharmaceutical budgets; and, with some delayed effect, the introduction of generics substitution [64].
As discussed, price and volume components influencing pharmaceutical expenditure have to be considered when analysing pharmaceutical expenditure. An increase in pharmaceutical expenditure resulting from the ‘price effect’ might be attributable to market entry of new expensive medicines, and ‘volume effect’ can be linked to demographic developments, such as an ageing population.
Further methodological challenges need to be addressed, including the scope of pharmaceutical expenditure.
Total pharmaceutical expenditure and sub-markets
Pharmaceutical expenditure may refer to the total market or to specific sub-markets. Major distinctions are public and private pharmaceutical expenditure, and out-patient and in-patient pharmaceutical expenditure.
Pharmaceutical expenditure from these sub-markets should ideally amount to total pharmaceutical expenditure. Other relevant sub-markets include the generics market, the prescription medicines market, the over-the-counter market, and the self-medication market. Expenditure data for a sub-market might be available for another sub-market, e.g. out-patient, only. For instance, pharmaceutical expenditure spent on generics is likely to be indicated only for the out-patient sector, for those medicines reimbursed by social health insurance, or both, because of limited data for other segments.
These gaps in data availability are also relevant to public/private pharmaceutical expenditure, and particularly out-patient/in-patient pharmaceutical expenditure. Some countries might have difficulties in indicating private pharmaceutical expenditure, or all components of it. Private pharmaceutical expenditure typically includes expenses of private households for non-reimbursed medicines, co-payments to reimbursable medicines, including co-payments under the reference price system, and expenses for medicines, which are, in principle, reimbursable but can be borne out-of pocket by the patient in specific cases; this may be because the price of the medicines is lower than the prescription fee. Although data for public pharmaceutical expenditure can easily be derived from the payers’ databases, separate data collection and assessment for private expenditure might be required. For instance, Bulgaria provides no information on private pharmaceutical expenditure, and what is indicated as total pharmaceutical expenditure in databases such as Eurostat is in fact public expenditure only.
Hospital (or in-patient) pharmaceutical expenditure is even more difficult to collect. It is not routinely surveyed at national levels. This is attributable to the financing system in European countries: pharmaceutical expenditure in hospitals are usually funded by hospital budgets [37]. As a result, what is indicated in the standard databases as total pharmaceutical expenditure refers, in practice, only to out-patient pharmaceutical expenditure. The definition of ‘hospital’ is challenged, as hospitals may also include hospital out-patient departments [23].
Data for total pharmaceutical expenditure in European countries as of 2009 are presented in Figure 4. For out-patient pharmaceutical expenditure, we referred to OECD health data [11]; if these were unavailable, Eurostat was used [12]. For hospital pharmaceutical expenditure, we conducted a separate survey. We asked competent authorities to provide hospital pharmaceutical expenditure data, or an estimate, for their countries. Some countries were able to provide the data; however, extra effort was required in most countries because the data were not easily available [18, 37]. Although we aimed to produce a complete picture for all 27 European Union Member States, representatives of several countries failed to provide these data, despite their efforts. Methodological difficulties and limitations are presented in Figure 4.
Out-patient pharmaceutical expenditure per inhabitant in European countries is shown in Figure 5. Here, a higher number of countries was able to provide data. In many cases, information on out-patient pharmaceutical expenditure was misinterpreted as total pharmaceutical expenditure, see Figure 5.
In addition, national pharmaceutical expenditure data cover expenditure for pharmaceuticals and further medical devises.
Other methodological challenges
Inter-country comparisons of pharmaceutical expenditure pose methodological challenges, specifically exchange rates and a possible weighting for the different economic situations of the countries. For the latter, use of the power purchasing parities (PPP) can eliminate the effects of differences in price levels between countries, thus reflecting the relative price level in relation of the purchasing power of a country [23, 65]. OECD, for instance, uses the PPP concept in their Health database, and they indicate pharmaceutical expenditure and other monetary indicators in US dollar PPP. In the European context, the use of Euros PPP would be preferred to US dollar PPP.
Another challenge is analysing the development of pharmaceutical expenditure over the years. It may be that the methodological approach applied to pharmaceutical expenditure data, even from the same source, might have changed at a particular time point for various reasons. This can explain breaks in the data series.
Finally, we would like to highlight the use of recent data. Many countries first publish preliminary expenditure data, based on estimations, and will ‘correct’ this estimate a few months later when all required data are available.
In this paper, we are not advising against the use of pharmaceutical expenditure data. Use of pharmaceutical expenditure data for analyses, and as a basis for policy decisions, is highly recommended despite limitations in the data sets. We must be aware of these limitations, however, and consider them when interpreting the data. As a minimum requirement, we advise checking the detailed notes accompanying the data set. One example is a note from the 2012 OECD Health at a Glance study: Pharmaceutical expenditure covers spending on prescription medicines and self-medication, often referred to as over-the-counter products. In some countries, the data also include other medical non-durable goods (adding approximately 5% to the spending). The expenditure also includes pharmacists’ remuneration when the latter is separate from the price of medicines. Pharmaceuticals consumed in hospitals are excluded (their inclusion would add another 15% to pharmaceutical spending approximately). Final expenditure on pharmaceuticals includes wholesale and retail margins and value-added tax [10]. The explanation is long but precise, and assists in understanding the coverage, quality and limitations of the data set.
In this paper, we show that pharmaceutical expenditure is influenced by several price and volume components. Therefore, policymakers are strongly recommended to focus on all aspects rather than on a single policy if they want to contain expenditure.
The interpretation and analysis of pharmaceutical expenditure can be compromised by limited availability of expenditure data in some segments. In some countries, pharmaceutical expenditure data refer to the public sector only. A particular challenge is the collection of hospital pharmaceutical expenditure which, as a rule, has not been routinely surveyed. What is presented under the heading ‘total pharmaceutical expenditure’ in many databases reflects only the out-patient sector. Research projects, such as PHIS, which aimed to fill gaps in data availability, provided valuable contributions to the evidence base.
Pharmaceutical expenditure is a major macroeconomic indicator for pharmaceutical and health-policy analyses. We support the use of this indicator but strongly advise studying which components are included in the given data set of pharmaceutical expenditure, and which are not, and considering the limitations appropriately in the analysis.
It is key for patients that policy makers base their decisions about policy measures on sound evidence. Pharmaceutical policy analysis is a major supportive tool for policy making. Analyses and forecasts, however, may be impaired by limitations in availability and comparability of data.
Although we acknowledge the perils of the pharmaceutical policy analyses as a result of methodological limitations and gaps in data availability, it should not defer us from undertaking such studies. Researchers and policy makers, however, are strongly advised to take caution when interpreting the data. In this study, we examined the components of pharmaceutical expenditure, which is a key indicator, and explored its pitfalls and limitations. By raising awareness and increasing knowledge of this indicator, we intend to contribute to better understanding and more careful interpretation of pharmaceutical expenditure data. This eventually benefits society as a whole, including patients.
We would like to thank the organizers of the EuroDURG/ISPE meeting ‘Better public health through pharmaco-epidemiology and quality use of medicine’ in Antwerp, Belgium, from 30 November to 3 December 2011, and particularly Dr Brian Godman, organizer of the session ‘Exploring units of expenditure in drug utilization studies’ on 1 December 2011 for inviting Dr Vogler to present on pharmaceutical expenditure and suggesting a paper based on the presentation.
We would like to thank our colleagues at the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies for surveying updated information on pharmaceutical policies. We also thank the members of the Pharmaceutical Pricing and Reimbursement Information (PPRI) network (http://whocc.goeg.at/Networks/ListOfMembers) for regularly providing information about changes in pharmaceutical policies in their countries. Further thanks go to the Pharma Price Information (PPI) service at the Austrian Health Institute for sharing price data.
The authors received no specific funding for this manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Nina Zimmermann, MA
Claudia Habl
WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH (Austrian Health Institute)/Geschäftsbereich ÖBIG, 6 Stubenring, AT-1010 Vienna, Austria
References
1. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Report. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG; 2008.
2. Rosian I, Antony K, Habl C, Vogler S, Weigl M. Benchmarking pharmaceutical expenditure. Cost-containment strategies in the European Union. Vienna: Österreichisches Bundesinstitut für Gesundheitswesen (ÖBIG); 2001.
3. OECD. Pharmaceutical pricing policies in a global market. Paris; 2008.
4. Carone G, Schwierz C, Xavier A. Cost-containment policies in public pharmaceutical spending in the EU. Brussels: European Commission, Directorate-General for Economics and Financial Afairs, 2012 [cited 2013 Jun 7]. Available from: http://ec.europa.eu/economy_finance/publications/economic_paper/2012/
pdf/ecp_461_en.pdf
5. Aaserud M, Dahlgren A, Kosters J, Oxman A, Ramsay C, Sturm H. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev. 2006;(2):CD005979.
6. Austvoll-Dahlgren A, Aaserud M, Vist G, Ramsay C, Oxman AD, Sturm H, et al. Pharmaceutical policies: effects of cap and co-payment on rational drug use. Cochrane Database Syst Rev. 2008;(1):CD007017.
7. Toumi M, Rémuzat C. EU Pharmaceutical expenditure forecast. Paris: Creativ_Ceutical. 2012.
8. Kanavos P, Vandoros S, Irwin R, Nicod E, Casson M. Differences in costs of and access to pharmaceutical products in the EU. Brussels: European Parliament, 2011.
9. Lu Y, Hernandez P, Abegunde D, Edejer T. The world medicines situation 2011—medicine expenditures. World Health Organization, Geneva. 2011.
10. OECD. Pharmaceutical Expenditure. In: Health at a glance: Europe 2012: OECD Publishing; 2012.
11. OECD. Health Data. Paris: OECD.
12. Eurostat. Eurostat Health Database.
13. OECD. Joint OECD-EUROSTAT-WHO Health Accounts Data-Collection Initiative Launched. OECD Health Update. 2006;2(2).
14. Gerdtham UG, Jönsson B. International comparisons of health expenditure: theory, data and econometric analysis. Handbook of Health Economics. 2000;1:11-53.
15. OECD. Health care reform. Controlling spending and increasing efficiency. Paris; 1994.
16. Or Z. Determinants of health outcomes in industrialised countries: a pooled, cross-country, time-series analysis. 2000. Report No:0255-0822.
17. Hoebert J, Mantel-Teuwisse A. PHIS Evaluation Report. Utrecht: Pharmaceutical Health Information System (PHIS); 2011.
18. PHIS. PHIS (Pharmaceutical Health Information System) database. [cited 2013 Jun 7]. Available from: https://phis.goeg.at/index.aspx?_nav0029
19. WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies. Pharmaceutical policy monitoring exercise with national competent authorities representated in the PPRI (Pharmaceutical Pricing and Reimbursement Information) network. Bi-annually, latest update: February 2013 (unpublished).
20. Gesundheit Österreich GmbH (Austrian Health Institute). Pharma Price Information (PPI) service. [cited 2013 Jun 7]. Available from: www.goeg.at/en/PPI
21. General Social Insurance Act § 351c, Federal Law No. 189/1955, latest change by Federal Law I No. 398/2011. 2012. German.
22. Bundesministerium für Gesundheit. Regelung für die Vorgehensweise der Preiskommission bei der Ermittlung des EU-Durchschnittspreises gemäß § 351c Abs. 6 ASVG. 1 January 2008.
23. WHO Collaborating Centre for Pharmaceutical. Pricing and Reimbursement Policies. Glossary of pharmaceutical terms. 2011. Available from: http://whocc.goeg.at/Literaturliste/Dokumente/MethodologyTemplate/PHIS%20Glossary_UpdatedApril2011.pdf
24. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe-A descriptive overview. Health Policy. 2012;104(1):50-60.
25. Leopold C, Mantel-Teeuwisse AK, Seyfang L, Vogler S, de Joncheere K, Laing RO, et al. Impact of External Price Referencing on Medicine Prices–A Price Comparison Among 14 European Countries. Southern Med Review. 2012;5(1).
26. Vogler S. How large are the differences between originator and generic prices? Analysis of five molecules in 16 European countries. Farmeconomia Health economics and therapeutic pathways. 2012;13(Suppl 3):29-41.
27. Godman B, Bucsics A, Burkhardt T, Haycox A, Seyfried H, Wieninger P. Insight into recent reforms and initiatives in Austria: implications for key stakeholders. Expert Rev Pharmacoecon Outcomes Res. 2008;8(4):357-71.
28. Kanavos P, Schurer W, Vogler S. Pharmaceutical distribution chain in the European Union: structure and impact on pharmaceutical prices. London/Vienna: EMINet/LSE/GÖG; 2011.
29. Vogler S, Habl C, Bogut M, Voncina L. Comparing pharmaceutical pricing and reimbursement policies in Croatia to the European Union Member States. Croat Med J. 2011;52(2):183-197.
30. Vogler S. Preisbildung und Erstattung von Arzneimitteln in der EU—Gemeinsamkeiten, Unterschiede und Trends. Pharmazeutische Medizin. 2012;14(1):48-56.
31. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240-9.
32. Ball D. Working paper 3: Regulation of mark-ups in the pharmaceutical supply chain—review series on pharmaceutical pricing policies and interventions. Geneva: World Health Organization and Health Action International; 2011.
33. Levison L, Laing R. The hidden costs of essential medicines. Essential Drugs Monitor. 2003;33:20-1.
34. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries–an overview. Generics and Biosimilars Initiative (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
35. Godman B, Bennie M, Baumgärtel C, Sovic-Brkicic L, Burkhardt T, Fürst J, et al. Essential to increase the use of generics in Europe to maintain comprehensive health care? Farmeconomia Health economics and therapeutic pathways. 2012;13(3S):
5-20.
36. Habl C, Vogler S, Leopold C, Schmickl B, FrÖschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich. Vienna: ÖBIG Forschungs- und Planungsgesellschaft mbH; 2008.
37. Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Pharmaceutical Health Information System (PHIS); 2010.
38. Vogler S, Zimmermann N, Mazag J. Procuring medicines in hospitals: results of the European PHIS survey. Eur J Hosp Pharm Prac. 2011(2):20-1.
39. Vogler S, Zimmermann N, Leopold C, Habl C, Mazag J. Discounts and rebates granted for medicines for hospital use in five European countries. The Open Pharmacoeconomics & Health Economics Journal. 2013;5:1-25.
40. Lopes S, Marty C, Berdai D. PHIS Pharma Profile France. Vienna: Pharmaceutical Health Information System (PHIS); 2011.
41. Vogler S, Zimmermann N, Habl C, Piessnegger J, Bucsics A. Discounts and rebates granted to public payers for medicines in European countries. South Med Rev. 2012;5(1):38-46.
42. Espín J, Rovira J, García L. Experiences and impact of European risk-sharing schemes focusing on oncology medicines. EMINet; 2011.
43. Adamski J, Godman B, Ofierska-Sujkowska G, Osinska B, Herholz H, Wendykowska K, et al. Risk sharing arrangements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. 2010;10:153.
44. European Pharmaceutical Marketing Research Association (EphMRA). EphMRA Lexicon. A pocket guide to pharmaceutical marketing and marketing research terms and definitions. Stalybridge, no year available.
45. World Health Organization. WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Introduction to drug utilization research. Oslo: World Health Organization; 2003.
46. World Health Organization. The rational use of drugs—Report of the conference of experts; 25-29 November 1985; Nairobi. 1987 [cited 2013 Jun 7]. Available from: http://apps.who.int/medicinedocs/documents/s17054e/s17054e.pdf
47. Lee D, Bergman U, Strom B. Studies of drug utilization. Pharmacoepidemiology. 1994;2:379-93.
48. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Policies to enhance prescribing efficiency in Europe: findings and future implications. Front pharmacol. 2010;1:141.
49. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
50. Vogler S, Schmickl B. Rationale Arzneimitteltherapie in Europa. Vienna: Gesundheit Österreich GmbH; 2010.
51. Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9(1):65-83.
52. Wettermark B, Pehrsson A, Juhasz-Haverinen M, Veg A, Edlert M, Tornwall-Bergendahl G, et al. Financial incentives linked to self-assessment of prescribing patterns: a new approach for quality improvement of drug prescribing in primary care. Qual Prim Care. 2009;17(3):179-89.
53. Elliot D, Byrne G. PPRI Pharma Profile Ireland. Vienna: Pharmaceutical Pricing and Reimbursement Information (PPRI); 2007.
54. Rosian I, Habl C, Vogler S. Pharmaceuticals market control in nine European countries. Vienna: Österreichisches Bundesinstitut für Gesundheitswesen (ÖBIG); 1998.
55. Leopold C, Habl C, Vogler S, Rosian-Schikuta I. Steuerung des Arzneimittelverbrauchs am Beispiel Dänemark. Vienna: Gesundheit Österreich GmbH (Austrian Health Institute); 2008.
56. Godman B, Paterson K, MalmstrÖm RE, Selke G, Fagot JP, Mrak J. Improving the managed entry of new medicines: sharing experiences across Europe. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):439-41.
57. DeSwaef A, Antonissen Y. PPRI Pharma Profile Belgium. Vienna: Pharmaceutical Pricing and Reimbursement Information (PPRI); 2008.
58. Fraeyman J, Van Hal G, Godman B, Beutels P. The potential influence of various initiatives to improve rational prescribing for proton pump inhibitors and statins in Belgium. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):141-51.
59. PPRI network members. PPRI /PHIS Pharma Profiles—country specific reports on pharmaceutical systems and policies. Vienna [cited 2013 Jun 7]. Available from: http://whocc.goeg.at/Publications/CountryReports, 2007-2012.
60. Vogler S, Zimmermann N, Leopold C, de Joncheere K. Pharmaceutical policies in European countries in response to the global financial crisis. South Med Rev. 2011;4(2):69-79.
61. Garuoliene K, Godman B, Gulbinovic J, Wettermark B, Haycox A. European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
62. Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):125-30.
63. Kullman D. PHIS Pharma Profile United Kingdom. Vienna: Pharmaceutical Health Information System (PHIS); 2011.
64. Czypionka T, Riedel M, RÖhrling G, Mayer S, Rasinger T. Mengenkontrolle im Arzneimittelbereich: internationale Evidenz für Österreich. Health System Watch. 2010(3):1-16. German.
65. Hill P. International price levels and purchasing power parities. OECD Economic Studies. 1986;6:133-59.
66. Zimmermann N, Vogler S. PHIS Hospital Pharma Report Austria. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
67. OECD. Health Data. Paris: OECD; June 2011.
68. Zalesakova P. PHIS Hospital Pharma Report Czech Republic. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
69. PHIS network members. Country-specific information provided to PHIS Hospital Pharma survey (unpublished).
70. Martinez V, Ferré de la Peña P, Izquierdo G, Lens Cabrera C. PHIS Pharma Profile Spain. Vienna: Pharmaceutical Health Information System (PHIS); 2010.
71. Närhi U. PHIS Hospital Pharma Report Finland. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
72. AIFA. PHIS Hospital Pharma Report Italy draft. Pharmaceutical Health Information System (PHIS); 2010 (unpublished).
73. Gulbe A, Innus J, Martinsons A. PHIS Hospital Pharma Report Latvia. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
74. Eurostat. Eurostat Health Database. February 2011.
75. Storms H, Schreurs M. PHIS Pharma Profile The Netherlands (draft, unpublished). Pharmaceutical Health Information System (PHIS); 2011.
76. Adamski J, Wndykowska K. PHIS Hospital Pharma Report Poland. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
77. Caldeira S, Furtado C, Vieira I, Baptista A. PHIS Hospital Pharma Report Portugal. Vienna: Pharmaceutical Health Information System (PHIS); 2010.
78. Aanes T, Ognøy AH, Festøy H. PHIS Hospital Pharma Report Norway. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
79. Zahra Pullis I. PHIS Hospital Pharma Report Malta. Vienna: Pharmaceutical Health Information System (PHIS); 2009.
|
Author for correspondence: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH (Austrian Health Institute)/Geschäftsbereich ÖBIG, 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2013 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/understanding-the-components-of-pharmaceutical-expenditure-overview-of-pharmaceutical-policies-influencing-expenditure-across-european-countries.html
|
Objective: To analyse similarities and differences between activities carried out by Austrian sickness funds to encourage more rational use of medicines, including increasing generics uptake. |
Submitted: 28 February 2013; Revised: 5 June 2013; Accepted: 7 June 2013; Published online first: 21 June 2013
Rational use of medicines should ensure that patients receive ‘medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community’ [1]. Core policies defined by World Health Organization (WHO) to promote rational use of medicines [2] include clinical guidelines, essential medicines lists, Drug and Therapeutics Committees (DTC), training in undergraduate curricula and continuing medical education, independent information on medicines and public education, avoidance of perverse financial incentives, and sufficient and sustainable government expenditure.
All European Union (EU) and European Economic Area (EEA) countries have reimbursement lists in place, usually in the form of positive lists [3–6], and Drug and Therapeutic Committees are a standard feature in European hospitals [7]. Public education programmes have been introduced in some countries [8], such as the antibiotics campaign in France [9] and the generics campaigns in France and Portugal [10–13].
Rational use of medicines, however, continues to be a challenge in many countries, including European countries [14]. The European Surveillance of Antibiotic Consumption project showed over- and misuse of antibiotics in European countries, with wide variation in type and volume of antibiotics prescribed [15]. Despite several interventions, patient adherence to medication remains poor [16–20]. Generic medicines help reduce the costs incurred by public payers [6, 21] and are, in accordance with the WHO Nairobi declaration [1], a way of supporting rational use of medicines. It seems, however, that generics policies are not fully exploited in some European countries [22–25]. Policies to promote generics uptake, which is defined as the increase in the use of generics compared with the originator or patent medicines within a substitutable class, include the promotion of generics substitution at all levels of the health system, and the promotion of generics acceptance by professionals, patients, and the general community [26–28].
Austria’s healthcare system is based on a social security system with compulsory insurance. The social insurance system includes the branches of health, accident and pension insurance, and, in 2012, consisted of 22 social security institutions. Within health care, nine regional sickness funds (for the nine Austrian provinces), six occupational sickness funds, and four further institutions, e.g. social security institution for farmers, provide public health insurance services for the Austrian population [29].
The pharmaceutical sector in Austria is organized in a two-tier system, with different funding mechanisms for primary care (outpatient, general practitioners and specialists) and hospital care. In primary care, funded medicines are included in the reimbursement list (Erstattungskodex). The Austrian Main Association of Social Insurance Institutions (MASSI), the umbrella organization of social insurance institutions, makes decisions about the inclusion of medicines into this national positive list after a pharmacological, therapeutic and health-economic evaluation. In the case of a positive decision, the sickness funds then fund the use of the listed medicines if prescribed by outpatient doctors, i.e. those working in the community care setting [30, 31]. Medicines in hospitals are financed out of hospital budgets and are, with some exemptions, included in the diagnosis related groups remuneration system. Social health insurance, however, co-funds inpatient medicines expenditure, as public and private non-profit hospitals for public benefit are financed by the provincial health funds, which receive funds from the federal government, the provinces, local authorities, and the social insurance institutions [32].
At the federal level, pharmaceutical policies strongly focus on pricing and reimbursement. In 2004, external price referencing for medicines to be included in the reimbursement list was introduced. It requires that the ex-factory price of these medicines cannot be higher than average prices in other EU Member States. The reimbursement system was reorganized, and a new reimbursement list (‘Reimbursement Code’) was launched [30, 31]. As a result of these reforms, prices can be controlled; however, pharmaceutical expenditure is still increased because of the volume component, e.g. inappropriate prescription possible over-or misuse of medicines [33].
In Austria, rational use of medicines is on the health policy agenda. For instance, the Federal Ministry of Health requested a study (published in 2008) to identify measures to control volume in Denmark compared with the situation in Austria [34]. In 2009, the multi-stakeholder working group on the rational use of medicines as part of the Pharmaceutical Committee was established as an advisory body to the Federal Ministry of Health, with the explicit aim of promoting rational use of medicines [35].
Austria has implemented a so-called ‘generic price link policy’ [36], i.e. it sets the prices of reimbursable generics and other ‘followers’ at a certain percentage lower than those of the originator, whose price subsequently also has to decrease [30]. Austria is the only country in the EU that has neither a reference price system, generics substitution nor International Nonproprietary Name (INN) prescribing in place [23]. Although a possible implementation of a reference price system and generics substitution has been discussed, a draft law to introduce such measures has not been passed [35].
Since 1999, the ‘Medicine and Reason’ initiative has been developing treatment recommendations for specific diseases, e.g. asthma and chronic obstructive pulmonary disease, type 2 diabetes, and rheumatoid arthritis, based on the evaluation of existing evidence. The recommendations are provided in the form of guidelines for doctors and patient leaflets. It started as a joint project between social insurance and the pharmaceutical industry. Doctor and pharmacist representatives became involved in 2003, thus ensuring broad stakeholder representation [37].
In social health insurance, responsibilities are shared between the federal entity, MASSI, and the sickness funds. MASSI decides on which medicines are selected for inclusion in the reimbursement list or remain included in the list, and at what price. This is imperative for the sickness funds. As payers, they have to cover the cost of reimbursable medicines prescribed by doctors under contract. The regional (and other) sickness funds act within the framework of decisions taken at the federal level. For instance, MASSI instigated prescribing restrictions, e.g. on statins [38], and on angiotensin receptor blocker [39], and decided to lift prescribing restrictions on generic losartan [40], with a view to enhancing the prescribing of generics; such measures could be followed up by the sickness funds.
Austrian sickness funds have also been exploring ways in which to enhance rational use of medicines, including an increased prescribing of generics. A more rational use of medicines is primarily promoted for public health reasons, but it can also contribute to savings for payers [3].
The objective of this study was to survey measures undertaken by Austrian sickness funds to enhance more rational use of medicines, including increasing generics uptake. Particularly, we aimed to analyse the variation in types of measures and target groups, and to explore differences and similarities among the approaches selected by the sickness funds. We did not include an impact assessment of the measures. The focus of this study was on initiatives launched and carried out by the sickness funds. A comprehensive description of the national reimbursement system for medicines in Austria [30–32, 41], and initiatives undertaken by MASSI [38–40, 42, 43], have been detailed elsewhere, and are outside the scope of this study.
We conducted a survey among selected sickness funds in Austria. We chose regional sickness funds that, in general, covered people in one Austrian province. We opted for regional sickness funds because they have larger and more mixed constituencies compared with other funds, e.g. those targeting specific professions. We selected those funds that were known to have been working on enhancing rational use of medicines. In accordance with the wishes of the Austrian Federal Ministry of Health, who commissioned the survey, we focused on sickness funds in eastern, central and southern parts of Austria. The findings of the survey among the four regional sickness funds are presented in this paper. This study was exploratory and therefore did not aim for complete coverage of all sickness funds in Austria.
All selected sickness funds have a pharmacoeconomic unit that aims to encourage doctors to prescribe more rationally. Staff within these pharmacoeconomic units were the primary contacts for information and data collection. We conducted face-to-face semi-structured interviews (average duration 2–3 hours) based on an interview guide in German, the official language in Austria. On request, the interview guide was shared with the interviewees in advance. The interview was structured thematically, and included activities targeted at prescribers, patients, and pharmacists. For each of the measures, we aimed to obtain information on experience with the measures, supporting or limiting factors, indicators for success, accompanying materials, and evaluations.
Between February and October 2010, we conducted interviews with responsible staff in the regional sickness funds of Burgenland (Burgenländische Gebietskrankenkasse, BGKK), Carinthia (Kärntner Gebietskrankenkasse, KGKK), Lower Austria (Niederösterreichische Gebietskrankenkasse, NÖGKK) and Upper Austria (Oberösterreichische Gebietskrankenkasse, OÖGKK), and gathered additional information provided by the sickness funds.
We compiled the findings and presented them to the data providers during an internal sickness funds meeting in spring 2011. We then circulated the document to the participating sickness funds for feedback and approval. In December 2012, they were asked to check the draft paper for accuracy of the measures listed.
The data surveyed refer to the year 2010. Updated information provided during the validation phase is considered in the discussion section.
The four sickness funds carried out several activities targeting their ‘contract doctors’, i.e. doctors in contractual relationship with them, to encourage them to prescribe more rationally. All sickness funds monitored the prescription behaviour of the doctors regularly and systematically, and used specific software tools to do this. Prescribers were provided information and feedback in different ways, e.g. written letters, personal meetings, and telephone conversations, and at different time intervals, see Table 1.
In encouraging doctors to prescribe more rationally, sickness funds set activities to motivate doctors to prescribe more generics. All sickness funds provided information on generics in different formats, see Table 1 and Table 2. For instance, letters sent to prescribers at regular intervals contained information about the rate of generics prescribed by the individual doctor compared with the average prescribing of generics in that region, and about possible savings that could have been achieved if the doctor had prescribed more generics alternatives. Educational events organised by sickness funds, often in cooperation with regional doctors’ associations, included generics as an agenda item. In training sessions for new contracted doctors, specific time slots were dedicated to pharmacoeconomic issues and prescribing of generics. Some sickness funds introduced benchmarking systems, financial incentives for contracted doctors to motivate them to prescribe generics, or both. A software system for use in doctors’ offices, which was offered by the sickness funds, allowed prescribers to identify the most economic therapeutic alternative.
Communication with pharmacists was much less intensive compared with prescribers. Informal meetings were organised between pharmacist representatives and sickness funds, see Table 3. Generics promotion was one topic in these talks.
Sickness funds tended to provide information for patients. These included a health journal targeted at patients, available in print and electronic format, public information events, media campaigns, leaflets and posters in doctors’ offices, and personal letters, see Table 4. A main aim of these campaigns was to raise awareness of generics among patients.
Following a recent change in legislation, sickness fund representation on the DTC in Austrian hospitals became mandatory. At the time of our survey, involvement was still relatively new. Furthermore, sickness funds launched capacity-building projects with the aim of improving dialogue between inpatient and outpatient care. These projects included training on rational use of medicines for junior doctors and nursing staff organised by representatives of the sickness funds at the hospital premises, see Table 5.
In addition to the activities carried out in their respective regions, sickness funds liaise with each other and with the federal entity MASSI. Pharmacoeconomic unit staff meet regularly to share and analyse data, exchange experience, and to discuss collaborations.
The sickness funds that we surveyed have been carrying out numerous activities to encourage rational use of medicines and generics uptake. We identified ‘core activities’ carried out by all funds. These included various measures targeting doctors to prescribe more rationally, including the prescription of more generics wherever available and appropriate, followed by prescription monitoring.
Several of the funds interviewed highlighted the training course offered by the regional sickness funds offered to prescribers who had recently entered into contractual relationship as an example of good practice. The training course was considered to be useful because it outlined the objectives of public payers. Interviewees highlighted the importance of offering new ‘contract doctors’ the opportunity of becoming acquainted with staff at the sickness funds to establish trust and encourage doctors to contact sickness funds with requests or concerns. Sickness fund staff believed that these training courses contributed to increased adherence to prescribing rules. This mirrors similar experience in other countries where educational activities have enhanced the relationship between payers and prescribers, and improved prescribing quality, for example, the provision of more information and prescribing activities to increase adherence to the Wise List, a list of recommended treatments in Stockholm County in Sweden [44].
Information-technology solutions and feedback to prescribers were identified as major elements of prescription monitoring. The Austrian sickness funds apply sophisticated information-technology programmes for monitoring the prescription pattern of doctors. All sickness funds use the same data warehouse [45], but the individual sickness funds only have access to their regional data. Currently, social health insurance in Austria applies these tools for monitoring prescription behaviour. They are not, however, intended to produce aggregated data for the purpose of comparisons at regional level for policymakers, researchers and the interested public, as in other countries. Most European countries have established nation-wide monitoring systems on pharmaceutical spending and consumption, and they provide publicly accessible national (and regional) statistics, e.g. Portugal, Italy, and England, [8, 46]. In Germany, in-depth scientific analyses of prescription data are, for instance, published in the annual Prescription Monitoring Report [47]. In Denmark, the online monitoring system ‘Ordiprax’ offers prescribers the possibility of accessing, at any time, their prescribing data and comparing them to the average prescribing patterns of doctors of the same specialty in their region [8, 34, 48]. Some Swedish counties offer similar solutions; for instance, the county of ÖstergÖtland established an online system that also allows doctors to benchmark their prescribing behaviour against colleagues [49].
In Austria, all the sickness funds that we surveyed tailored feedback to doctors. The pharmacoeconomic units of the sickness funds send letters out to prescribers at regular intervals. These contain information about doctors’ prescriptions and, in some cases, compare them to average prescribing patterns in the region. In some sickness funds, staff telephone doctors to follow up these letters. Despite these core activities of letters and discussions with doctors, our survey showed wide variance in the implementation and design of the measures between the individual sickness funds. Respondents confirmed different approaches in their communication strategies to prescribers, insured parties and partners, e.g. regional doctors’ associations. These differences most likely stem from the different environments in which the sickness funds operate: what works well in a rural, less densely populated area is not necessarily successful in provinces with more urban centres. An example of a coordinated, yet differentiated approach is the collaboration between sickness funds on their monthly health journal sent to prescribers. Representatives of the pharmacoeconomic units form a joint editorial committee whose members discuss and define the outline of each edition. Each sickness fund then produces its own journal by considering the particulars of their constituency. These variations in strategies mirror evidence from other European countries about different regional approaches in the design of measures [50].
Another outcome of the survey was that evidence-based medicine plays an important role in the work of the pharmacoeconomic units. Staff within the funds forward economically founded arguments, e.g. possible cost savings in case of the prescription of a less expensive but equivalent generic drug, in their communication with prescribers, and they supplement these statements with the latest scientific findings. They also provide news and summaries of relevant peer-reviewed articles. As such, the sickness funds act as providers of independent medicines information that would require time and resources if doctors had to search for the information independently [51]. Supportive evidence-based data are also intended to reduce prescribers’ reluctance to prescribe generics. A study in Germany, a country with a much higher generics uptake than Austria, showed that primary care doctors were still skeptical about prescribing generics [52]. Although the focus of our analysis was rather on activities related to generics promotion, some information and educational activities provided by sickness funds also focused on new medicines and polypharmacy, e.g. the initiative of the ‘medicine passport’ in Carinthia listing all medicines dispensed to the patient in a booklet.
Recently, some sickness funds have started to offer similar educational activities and information to private doctors. In fact, the sickness fund of Upper Austria initiated this after the survey period. We are not aware of similar approaches in other countries. Despite the lack of evidence on the possible effect of such a measure, we consider the comprehensive strategy as a promising approach to encourage rational use of medicines.
One of the major challenges in Austria’s healthcare policy is dual financing, i.e. different payers for medication in the outpatient sector (sickness funds) and in the inpatient sector (hospital owners, e.g. provinces). This creates specific incentives. In order to minimize their own costs, healthcare providers and public payers may try to transfer patients and costs between the settings. This may be the case for high-cost medicines, which pharmaceutical companies might seek to launch in the hospital sector where no price control is applied [31]. Other medicines, particularly cardiovascular medicines and other treatments for chronic diseases, are provided at low or no cost to hospitals [53–56]. Studies have confirmed how medication prescribed during the hospital stay can influence future medicines prescribed [57–59]. Therefore, the surveyed sickness funds have opened a dialogue with doctors in hospitals to discuss more rational prescribing, including the option of prescribing generics. This is a voluntary activity, as sickness funds have no mandate for hospitals and cannot hold the doctors in hospitals accountable for what they prescribe. Given the challenges on interface management, the sickness funds thereby responded to an urgent need to improve the cooperation between primary and hospital care. Findings from a project carried out by the sickness fund in the province of Burgenland showed that improved cooperation between hospital doctors, primary care doctors and sickness funds, enhanced prescribing of generics [60]. Another important initiative for medicines management at the interface is statutory participation (without voting rights) of sickness funds in every hospital via representation on the DTC. This measure had just been introduced at the time of the survey. Interview partners who had already attended DTC meetings, at the time of the survey, reported more fruitful insights into the hospital policy and activities; representation of sickness funds on the DTCs apparently contributed to a better joint understanding.
Further measures in Austria are, however, needed to enhance interface management, e.g. joint reimbursement lists and joint DTC for outpatient and hospital care as demonstrated in Sweden [44] and Scotland [61]. This would, however, require a fundamental change in the organization and funding of the Austrian healthcare and pharmaceutical sector, which is beyond the mandate of the sickness funds.
A similar picture can be seen for all sickness funds. Most measures address doctors to encourage them to prescribe more rationally. The second important target group is the insured party who is typically addressed through information activities (journal, information events). Few measures, if any, targeted pharmacists, despite evidence showing the important role of pharmacists in enhancing more rational use of medicines in several countries [19, 24, 62–64]. The reason why pharmacists in Austria are not considered a relevant target group by sickness funds may be because pharmacists cannot play an important role in generics policies, as Austria is the only country in the EU where neither generics substitution nor INN prescribing are permitted nor is a reference price system in place [23]. Pharmacists do, however, play an important role in promoting rational use of medicines in other areas, e.g. pharmaceutical care and medication safety. Another reason that pharmacists have a lower priority than doctors is that pharmacoeconomic units in sickness funds employ few staff. They have to make choices on where to make the difference, and this will be, in economic terms, the prescribers.
Finally, it should be acknowledged that all activities carried out by sickness funds to encourage a more rational use of medicines are influenced by the underlying legal framework. The ‘guidelines on economic prescription of medicines and therapeutic aids’, which ask for the prescription of the least expensive equivalent medicine, can be considered a supporting factor. The lack of regulation on generics promotion regulation (no INN prescribing, no generics substitution, no reference price system [23]) is likely to be a barrier for sickness funds, because they have to motivate doctors to prescribe generics whereas, in other countries with mandatory generics substitution this would not be an issue. It has been shown that generics substitution or INN prescribing, particularly if introduced as a mandatory measure, has a positive effect on generics uptake and lowering of prices [24, 25, 36, 65–73]. Furthermore, patients in Austria are not offered incentives to ask explicitly for generics. A pilot study with a company sickness fund in Austria proved that a partial reimbursement of the prescription fee in case of a generic being dispensed led to an increased share of generic prescription and a reduction in pharmaceutical expenditure of that sickness fund [74]. Given the lack of national statutory generic promotion policies, activities provided by the sickness funds include a variety of ‘voluntary’ projects. This is not a new development; a decade ago, the sickness fund BGKK ran a project to increase the prescribing of generic ACE inhibitors in 2000. This resulted in considerable savings for the sickness fund [75].
BGKK was also involved in pilot projects evaluating specific diagnostic tests, as it was believed that a more detailed and reliable diagnosis could support the identification of the best treatment and also contribute to reduced pharmaceutical expenditure. A sample of 30 BGKK’s contracted doctors tested the application of an immunologic rapid antigen detection test for Group A Streptococci, which helped to distinguish bacterial infection from viral infections of the upper respiratory tract. Subsequent prescribing of antibiotics was compared with the one prescription pattern of nearly all other remaining contracted general practitioners who did not have access to the test. The study [76] showed that use of the test increased diagnostic accuracy and improved appropriate prescribing of antibiotics. With these findings and results from other studies, see Table 2, [77, 78], BGKK was able to contribute evidence to the scientific community. No other sickness fund provided such data.
Our study was conducted in 2010. We know that the measures listed continue to be undertaken and have even expanded. Cooperation between sickness funds and hospitals has increased because of sickness fund representation on the DTC and some further projects. For instance, in Upper Austria, patient discharge letters indicate the name of the active ingredient instead of the specific brand name, and hospitals procure defined generics that are likely to be used in outpatient treatment.
We acknowledge possible shortcomings in the findings owing to the selected methodology of qualitative interviews. Some measures, however relevant, might not have been listed by a sickness fund. We are confident that we have covered all major activities, as the measures were compiled and shared with the data providers for feedback. It allowed us to verify the accuracy of the information.
Even if the survey does not comprehensively cover all Austrian sickness funds, we believe that the survey is of value because it provides information about the strategies used by sickness funds to improve rational use of medicines. It may, therefore, serve as a starting point for reflections on how to continue enhancing rational use and generics promotion in future.
This survey showed that numerous activities are being implemented by Austrian sickness funds to encourage more rational use of medicines. Most initiatives target contracted doctors to motivate them to prescribe rationally. In particular, sickness funds encourage doctors to prescribe more generics. Sickness funds are required to apply this strategy because neither INN prescribing nor generics substitution is in place in Austria to support generics uptake.
We suggest stronger involvement of pharmacists. Pharmacists could play a vital role in generics promotion, particularly by contributing to an improved image of generics. Although more measures to encourage rational use of medicines are welcome, we acknowledge limited staff resources within the sickness funds may make this difficult. The findings suggest that the pharmacoeconomic units provide activities over and above their specified remits.
A key challenge in the Austrian healthcare system is cooperation between the outpatient and hospital sectors which is disincentivized by a dual financing scheme. Sickness funds responsible for primary care are now targeting hospitals with their information and educational activities. These are important initiatives, but further policies would be required at national level, e.g. a joint DTC, to improve pharmacotherapy at the interface between primary care and hospitals.
At the national level, a publication of pharmaceutical spending and consumption monitoring is currently lacking. It is recommended exploring ways in which to implement a nation-wide monitoring system.
The sickness funds apply a range of tools to enhance more rational use of medicines, including improving generics uptake. No evaluation of the effect of these policies has yet been published. Further research is required to analyse the effectiveness of the measures.
We are very grateful to the members of the four sickness funds presented: DI Berthold Reichardt (Burgenländische Gebietskrankenkasse); Dr Margot Reiter, Dr Ursula Riess, Dr Bernadette MÖrtl-Kessler (Kärntner Gebietskrankenkasse); Herbert Feichter, Dr Michaela Stitz, Dr Jana Fischer, Msc (NiederÖsterreichische Gebietskrankenkasse); and Dr Anna Labek, MPH, Mag Gerhard Arzt (OberÖsterreichische Gebietskrankenkasse).
They were available for face-to-face interviews, provided a wealth of information and materials, and checked the accuracy of the measures and activities listed. This paper would never have been possible without their contributions.
Furthermore, we would like to thank all members of the group Argumentationsgruppe Heilmittel, in which the interview partners of the surveyed sickness funds were represented, for their interest in the study and for encouraging us to produce this manuscript.
We also thank our colleague Ms Bettina Schmickl who supported this study by participating in some of the interviews and reporting them.
Finally, our thanks go to Dr Wolfgang Ecker of the Austrian Federal Ministry of Health who commissioned Gesundheit Österreich GmbH to conduct this study.
It is important that public payers take action to meet the policy goals of sustainable funding and encourage a more rational use of medicines, including prescribing of generic medicines. In this paper, we review the activities carried out by sickness funds in Austria and provide evidence that successful practices can be implemented at regional level. A possible mix of policies and activities could be undertaken to contribute to more rational use of medicines. Although more rational use of medicines may have some cost-containment implications for payers, it benefits, first and foremost, patients by increasing patient safety and adherence.
The survey with the sickness funds was funded by the Austrian Federal Ministry of Health. No specific funding was provided for the writing of the article.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Nina Zimmermann, MA
WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH (Austrian Health Institute)/Geschäftsbereich ÖBIG, 6 Stubenring, AT-1010 Vienna, Austria
References
1. World Health Organization. The rational use of drugs. Report of the conference of experts, Nairobi, 25-29 November 1985. 1987 [cited 2013 Jun 5]. Available from: http://apps.who.int/medicinedocs/documents/s17054e/s17054e.pdf
2. World Health Organization. Promoting rational use of medicines: core components. WHO Policy Perspectives on Medicines. 2002;5:1-6.
3. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Report. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG; 2008.
4. Vogler S, Espin J, Habl C. Pharmaceutical Pricing and Reimbursement Information (PPRI) – New PPRI analysis including Spain. Pharmaceuticals Policy and Law. 2009;11(3):213-34.
5. Vogler S, Habl C, Bogut M, Voncina L. Comparing pharmaceutical pricing and reimbursement policies in Croatia to the European Union Member States. Croat Med J. 2011;52(2):183-197.
6. Carone G, Schwierz C, Xavier A. Cost-containment policies in public pharmaceutical spending in the EU. Brussels: European Commission, Directorate-General for Economics and Financial Afairs, 2012 [cited 2013 Jun 5]. Available from: http://ec.europa.eu/economy_finance/publications/economic_paper/2012/pdf/ecp_461_en.pdf
7. Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Pharmaceutical Health Information System; Gesundheit Österreich GmbH / Geschäftsbereich ÖBIG; 2010.
8. Vogler S, Schmickl B. Rationale Arzneimitteltherapie in Europa. Vienna: Gesundheit Österreich GmbH; 2010.
9. Sabuncu E, David J, Bernède-Bauduin C, Pépin S, Leroy M, Boëlle P-Y, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS medicine. 2009;6(6):e1000084.
10. Godman B, Bennie M, Baumgärtel C, Sovic-Brkicic L, Burkhardt T, Fürst J, et al. Essential to increase the use of generics in Europe to maintain comprehensive health care? Farmeconomia Health economics and therapeutic pathways. 2012;13(3S):5-20.
11. Sermet C, Andrieu V, Godman B, Van Ganse E, Haycox A, Reynier JP. Ongoing pharmaceutical reforms in France: implications for key stakeholder groups. Appl Health Econ Health Policy. 2010;8(1):7-24.
12. Lopes S, Marty C, Berdai D. PHIS Pharma Profile France. Vienna: Gesundheit Österreich GmbH / Geschäftsbereich ÖBIG; 2011.
13. Habl C, Vogler S, Leopold C, Schmickl B, FrÖschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich.Vienna, 2008.
14. Holloway K, Van Dijk L. Rational use of medicines. In: World Health Organization (WHO), Editors. The World Medicines Situation 2011. Geneva; 2011.
15. Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother. 2006;58(2):401-7.
16. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011.
17. Hughes DA, Bagust A, Haycox A, Walley T. The impact of non-compliance on the cost-effectiveness of pharmaceuticals: a review of the literature. Health Econ. 2001;10(7):601-15.
18. van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7(1):55.
19. Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Effectiveness of Interventions by Community Pharmacists to Improve Patient Adherence to chronic medication: a systematic review. Ann Pharmacother. 2005;39(2):319-28.
20. Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of Clinical Pharmacy and Therapeutics. 2001;26(5):331-42.
21. Godman B, Wettermark B, Bishop I, Burkhardt T, Fürst J, Garuoliene K. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
22. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Conference 2007. Agenda. PPRI Conference; 29.05.2012; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2007.
23. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries-an overview. Generics and Biosimilars Initiative (GaBI) Journal. 2012;1(2):93–100. doi:10.5639/gabij.2012.0102.020
24. Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):59-72.
25. Vitry A, Martin A, MPharmS BG, Sermet C, Herholz H, Bishop I, et al. Payers endorse generics to enhance prescribing efficiency: impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2012.0102.017
26. World Health Organization. Technical Brief Series – Brief No 1: Improving access to essential medicines and creating efficiencies by encouraging the uptake of generic medicines. [cited 2013 Jun 5]. Available from: http://www.who.int/healthsystems/topics/financing/healthreport/GenericsTBNo1.pdf
27. Cameron A, Laing R. Cost savings of switching private sector consumption from originator brand medicines to generic equivalents. World Health Report, Background Paper, 35. 2010 [cited 2013 Jun 5]. Available from: http://www.who.int/healthsystems/topics/financing/healthreport/35MedicineCostSavings.pdf
28. World Health Organization, Health Action International (HAI). Measuring medicine prices, availability, affordability and price components. 2nd edition. Geneva, 2008.
29. Habl C, Bachner F, Klinser D, Ladurner J. The Austrian Health Care System. Key Facts. Vienna: Austrian Federal Ministry of Health; 2010.
30. Leopold C, Habl C, Vogler S, Morak S. PPRI Pharma Profile Austria. Vienna: Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG), commissioned by the European Commission, DG Public Health and Consumers and the Austrian Federal Ministry of Health; 2008.
31. Godman B, Bucsics A, Burkhardt T, Haycox A, Seyfried H, Wieninger P. Insight into recent reforms and initiatives in Austria: implications for key stakeholders. Expert Rev Pharmacoecon Outcomes Res. 2008;8(4):357-71.
32. Zimmermann N, Vogler S. PHIS Hospital Pharma Report Austria. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG; 2009.
33. Czypionka T, Riedel M, RÖhrling G, Mayer S, Rasinger T. Mengenkontrolle im Arzneimittelbereich: internationale Evidenz für Österreich. Health System Watch. 2010(3):1-16. German.
34. Leopold C, Habl C, Vogler S, Rosian-Schikuta I. Steuerung des Arzneimittelverbrauchs am Beispiel Dänemark. Vienna: Gesundheit Österreich GmbH (Austrian Health Institute); 2008.
35. Hlava A, Bednar W, Birner A, Bronneberg G, FülÖp G, Grabner I, et al. Gesundheitsbericht an den Nationalrat 2009: Berichtszeitraum 2005-2007 [Health Report to Parliament 2009. Reporting period 2005-2007; report in German]. Vienna: Federal Ministry of Health and Gesundheit Österreich GmbH (Austrian Health Institute); 2009.
36. Vogler S. How large are the differences between originator and generic prices? Analysis of five molecules in 16 European countries. Farmeconomia Health economics and therapeutic pathways. 2012;13(Suppl 3):29-41.
37. Initiative Arznei & Vernunft. Vernünftiger Umgang mit Medikamenten. [cited 2013 Jun 5]. Available from: http://www.arzneiundvernunft.info/
38. Godman B, Burkhardt T, Bucsics A, Wettermark B, Wieninger P. Impact of recent reforms in Austria on utilization and expenditure of PPIs and lipid-lowering drugs: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2009;9(5):475-84.
39. Voncina L, Strizrep T, Godman B, Bennie M, Bishop I, Campbell S, et al. Influence of demand-side measures to enhance renin-angiotensin prescribing efficiency in Europe: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):469-79.
40. Bucsics A, Godman B, Burkhardt T, Schmitzer M, MalmstrÖm RE. Influence of lifting prescribing restrictions for losartan on subsequent sartan utilization patterns in Austria: implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2012;12(6):809-19.
41. Zimmermann N, Vogler S. PPRI/PHIS Pharma Profile Austria (draft, unpublished). Pharmaceutical Pricing and Reimbursement Information (PPRI)/Pharmaceutical Health Information System (PHIS). Vienna: Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG); 2012.
42. Godman B, Bucsics A, Burkhardt T, Schmitzer M, Wettermark B, Wieninger P. Initiatives to enhance renin–angiotensin prescribing efficiency in Austria: impact and implications for other countries. Expert Rev Pharmacoecon Outcomes Res. 2010;10(2):199-207.
43. MalmstrÖm R, Godman B, Diogene E, Baumgaertel C, Bennie M, Bishop I, et al. Dabigatran – a case history demonstrating the need for comprehensive approaches to optimize the use of new drugs. Front Pharmacol. 2013;4:39.
44. Gustafsson LL, Wettermark B, Godman B, Andersén-Karlsson E, Bergman U, HasselstrÖm J, et al. The ‘wise list’- A comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108(4):224-33.
45. Ganjeizadeh-Rouhani A. Business Intelligence im Gesundheitswesen. Soziale Sicherheit. 2010 (July/August):366-71.
46. Kullman D. Vienna: Pharmaceutical Health Information System (PHIS); 2011. Pharma Profile United Kingdom.
47. Schwabe U, Paffrath D. Arzneiverordnungs-Report 2012: Aktuelle Daten, Kosten, Trends und Kommentare. Springer; 2012.
48. Thomsen E, Er S, Rasmussen PF. PPRI Pharma Profile Denmark. Vienna: Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG); 2008.
49. Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res.2009;9(1):65-83.
50. Saltman RB, Busse R, Figueras J. Social health insurance systems in western Europe. Maldenhead, New York; 2004.
51. Schaaber J, Kochen MM, Müller-Oerlinghausen B, Niebling W. Warum unabhängige Arzneimittelzeitschriften und Fortbildungsveranstaltungen wichtig sind. In: Lieb K, Klemperer D, Ludwig W-D, Eds. Interessenkonflikte in der Medizin: Springer Berlin Heidelberg; 2011. p. 237-52.
52. Simmenroth-Nayda A, Hummers-Pradier E, Ledig T, Jansen R, Niebling W, Bjerre LM, et al. Prescription of generic drugs in general practice. Results of a survey of general practitioners. Med Klin. 2006;101(9):705-10. German.
53. Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Commissioned by the European Commission, Executive Agency for Health and Consumers (EAHC) and the Austrian Federal Ministry of Health (BMG); 2010.
54. Langebner T. Managing drug costs in Austria. Eur J Hosp Pharm Prac. 2007;13(5):79.
55. de Vries CS, van Diepen NM, Tromp TF, de Jong-van den Berg LT. Auditing GPs’ prescribing habits: cardiovascular prescribing frequently continues medication initiated by specialists. Eur J Clin Pharmacol. 1996;50(5):349-52.
56. Vogler S, Zimmermann N, Leopold C, Habl C, Mazag J. Discounts and Rebates Granted for Medicines for Hospital Use in Five European Countries. The Open Pharmacoeconomics & Health Economics Journal. 2013(5). Epub ahead of print.
57. Gallini A, Legal R, Taboulet F. The influence of drug use in university hospitals on the pharmaceutical consumption in their surrounding communities. Br J Clin Pharmacol. 2013 Apr;75(4):1142-8.
58. Feely J, Chan R, McManus J, O’Shea B. The influence of hospital-based prescribers on prescribing in general practice. Pharmacoeconomics. 1999;16(2):175-81.
59. Fahey T, Sinclair H. Hospital initiated prescribing in the General Medical Services scheme. Ir Med J. 1993;86(4):122-4.
60. Wolzt M, Ohrenberger G, Reichardt B. Cost reduction with project based prescription of generic ACE inhibitors. Wien Klin Wochenschr. 2003;115(1-2):23-8. German.
61. Herms S, Rutledge P. The Lothian Joint Formulary: overcoming the barriers to formulary success. Pharmacy in Practice. 2002;12(6):279-85.
62. Vogler S, Arts D, Habl C. Community Pharmacy in Europe. Lessons from deregulation – case studies. Vienna: Österreichisches Bundesinstitut für Gesundheitswesen (ÖBIG); 2006.
63. Clifford S, Barber N, Elliott R, Hartley E, Horne R. Patient-centred advice is effective in improving adherence to medicines. Pharm World Sci. 2006;
28(3):165-70.
64. Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond C, et al. Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database of Syst Rev. 2010;(7).
65. Redman T, KÖping HÖggärd M. PPRI Pharma Profile Sweden.
66. Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):125-30.
67. Godman B, Shrank W, Andersen M, Berg C, Bishop I, Burkhardt T, et al. Policies to enhance prescribing efficiency in Europe: findings and future implications. Front pharmacol. 2010;1:141.
68. Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing? Br J Clin Pharmacol. 2010;70(3):335-41.
69. Simoens S. The Portuguese generic medicines market: a policy analysis. Pharmacy Practice. 2009;7(2):74-80.
70. Godman B, Shrank W, Wattermark B, Andersen M, Bishop I, Gustafsson LL. Use of generics – a critical cost containment measure for all healthcare professionals in Europe? Pharmaceuticals. 2010;3(8):2470-94.
71. Vogler S, Zimmermann N. The potential of generics policies: more room for exploitation–PPRI Conference Report. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):146-9. doi:10.5639/gabij. 2012.0103-4.030
72. Aalto-Setälä V. The impact of generic substitution on price competition in Finland. Eur J Health Econ. 2008;9(2):185-91.
73. Garuoliene K, Godman B, Gulbinovic J, Wettermark B, Haycox A. European countries with small populations can obtain low prices for drugs: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
74. Gouya G, Reichardt B, Bidner A, Weissenfels R, Wolzt M. Partial reimbursement of prescription charges for generic drugs reduces costs for both health insurances and patients [Article in German]. Wien Klin Wochenschr. 2008;
120(3-4):89-95. German.
75. Rosian I, Vogler S. Generika Modell Burgenland. Wien: Österreichisches Bundesinstitut für Gesundheitswesen; 2001. German.
76. Reichardt B, PichlhÖfer O, Zehetmayer S, Maier M. Current diagnosis of acute pharyngitis. [Article in German]. Wien Med Wochenschr. 2009;
159(7-8):202-6. German.
77. Hoffmann K, Reichardt B, Zehetmayer S, Maier M. Evaluation of the implementation of a rapid streptococcal antigen test in a routine primary health care setting. Wien Klin Wochenschr. 2012;124(17-18):633-8.
78. Hoffmann K, Leifheit AK, Reichardt B, Maier M. The antibiotic prescription and redemption gap and opportunistic CRP point-of-care testing. A cross-sectional study in primary health care from Eastern Austria. Wien Klin Wochenschr. 2013:1-6.
|
Author for correspondence: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH (Austrian Health Institute)/Geschäftsbereich ÖBIG, 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2013 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Related article
National and regional activities by sickness funds in Austria to encourage the rational use of medicines
Source URL: https://gabi-journal.net/how-do-regional-sickness-funds-encourage-more-rational-use-of-medicines-including-the-increase-of-generic-uptake-a-case-study-from-austria.html
|
Abstract: |
Submitted: 16 September 2012; Revised: 17 September 2012; Accepted: 19 September 2012; Published online first: 21 September 2012
Dylst et al. show that a reference price system is a broadly used policy in European countries: the majority of the EU Member States have implemented such a system [1].
A key sentence by Dylst et al. is: ‘Unlike its name suggests, a reference pricing system is not a pricing system, but in fact a reimbursement system.’ This statement is key and contributes to a better understanding of this policy. The authors correctly define a reference price system as ‘a system that establishes a reimbursement level or reference price for a group of interchangeable medicines’: terminology clarity is important also because reference price systems are sometimes confused with a pricing policy called external price referencing. That policy is a ‘practice of using the price(s) of a medicine in one or several countries in order to derive a benchmark or reference price for the purposes of setting or negotiating the price of the product in a given country’ [2], and it is also very common in European countries [3].
As a reimbursement policy, a reference price system requires a specific design, in particular with regard to the clustering of the reference groups and the definition of the reimbursement limits (reference limits). The article describes the different approaches to organizing the reference price systems in the European countries and their possible consequences. Dylst et al. highlight that different approaches are recommended for different environments: while, in principle, the relationship ‘the lower reference price, the higher savings’ appears logical, there are settings, particularly in developing generics markets, where a higher reference price appears to achieve better results. A general lesson for policymakers can be drawn: when implementing policy measures, the context needs to be taken into consideration, and, though it is important to learn from the experiences of other countries, successful policy measures cannot be simply copied from one country to another but have to be adapted to the country specific environment [4].
Further, good timing and implementation planning is important when this policy measure is introduced or further developed. Regarding generics substitution, World Health Organization strongly recommends introducing this policy as a voluntary measure, i.e. legal framework allowing it; then encouraging it, e.g. via incentives, and in a final stage having mandatory generics substitution [5]. The implementation of a reference price system should follow a similar approach: the clusters should be defined in a more limited way in the beginning [Anatomical Therapeutic Chemical (ATC)-level 5] and can, after experience is gained, be broadened over the course of time (to the ATC-level 4 and even ATC-level 3).
This approach is recommended firstly because it facilitates the technical process for the staff administrating the system and secondly because stakeholders are more likely to accept the reference price system during a phased introduction. The stakeholder aspect was not addressed by Dylst et al. However, bringing stakeholders on board, as early as possible, is a major prerequisite for a successful policy implementation. The implementation of a reference price system, particularly when introduced in connection with generics substitution and/or with prescribing by the international non-proprietary name, may raise concerns to physicians who might be concerned about a loss of their ‘therapeutic freedom’. A good communication policy could facilitate the acceptance by stakeholders of the measures introduced. A study from Greece suggested that doctors would be willing to prescribe more generically if there was a clear generics promotion policy [6]. Having said this, it must be pointed out that the reference price system is a policy which has the potential to increase possible conflicts between prescribers and pharmacists. Nonetheless, good practice examples exist: a successful cooperation between the two groups was reported from Denmark where pharmacists and prescribers stressed the importance of having a clear understanding of their roles in the system which they consider as complementary and not conflicting [7].
Dialogue and information are therefore both of major importance also, and especially, when addressing the concerns of patients. In a study performed to explore prerequisites for successful implementation of a reference price system in Austria [8] (where it has yet not been introduced), case studies highlighted that in some European countries difficulties were encountered after the introduction of a reference price system because rumours about the low quality of generics had been spread and were not responded to appropriately. As a result, patients were confused and concerned.
Dylst et al. also addressed the issue of socio-economic equity concerns related to reference price systems. Though there are few studies on this topic available, evidence could dispel concerns about possible inequity. In fact, patients might even become strong partners in supporting generics policies. They have the purchasing power to ask for generics if incentivized to do so. But they need to understand the policy. Therefore, a policy providing clear information to patients about the function and benefits of a reference price system and about generics in general is required.
Reference price systems have been introduced in several European countries as a reimbursement policy. All of these have a publicly funded health and pharmaceutical system (either social health insurance or national health service). A recently published article on pro-generic drug policies in low- and middle-income countries (LMIC) also described reference pricing systems [9]. Because of the continuing investment into insurance systems in LMIC, reference pricing systems are a policy option that must be explored by these countries. The prerequisites for successful implementation (appropriate design of the system, stakeholder involvement, and an information policy) are the same as in European countries.
The editorial refers to the article of Dylst et al. whose findings suggest that reference price systems generate savings for healthcare budgets in the short term without a negative impact on the health of patients. This editorial highlights the role of stakeholders, including patients. Patients can become strong partners in supporting reference price systems and other generic drug policies but they need to have a good understanding of the policies. Therefore, a policy providing clear information to patients was stressed as key in the editorial.
Competing interests: None.
Provenance and peer review: Commissioned; internally peer reviewed.
References
1. Dylst P, Vulto A, Simoens S. Reference pricing systems in Europe: characteristics and consequences. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):127-31. doi:10.5639/gabij.2012.0103-4.028
2. Folino-Gallo P, Muscolo L, Vogler S, Morak S. PHIS Glossary: Glossary for pharmaceutical policies/systems developed in the Pharmaceutical Health Information System (PHIS) Project. 2009, latest update of print version: 2011; regularly updated online. Vienna: WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies; 2011.
3. Leopold C, Vogler S, Mantel-Teeuwisse AK, de Joncheere K, Leufkens HG, Laing R. Differences in external price referencing in Europe: a descriptive overview. Health Policy. 2012;104(1):50-60. Epub 2011 Oct 19.
4. Vogler S, Habl C, Leopold C, Rosian-Schikuta, et al. PPRI Report. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG; 2008.
5. World Health Organization [homepage on the Internet]. How to develop and implement a national drug policy. 2nd ed. Geneva: World Health Organization; 2001. [cited 2012 Sep 17]. Available from: http://apps.who.int/medicinedocs/en/d/Js2283e/
6. Tsiantou V, Zavras D, Kousoulakou H, Geitona M, Kyriopoulos J. Generic medicines: Greek physicians’ perceptions and prescribing practices. J Clin Pharm Ther. 2009;34(5):547-54.
7. Leopold C, Habl C, Vogler S, Rosian-Schikuta I. Steuerung des Arzneimittelverbrauchs am Beispiel Dänemark. Gesundheit Österreich GmbH. 2008 Dec. German.
8. Habl C, Vogler S, Leopold C, Schmickl B, Fröschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich. ÖBIG Forschungs- und Planungsgesellschaft mbH. 2008 Feb. German.
9. Kaplan WA, Ritz LS, Vitello M, Wirtz VJ. Policies to promote use of generic medicines in low and middle income countries: a review of published literature, 2000–2010. Health Policy. 2012;106(3):211-24.
|
Author: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG – Austrian Health Institute, 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Related article
Reference pricing systems in Europe: characteristics and consequences
Source URL: https://gabi-journal.net/reference-price-systems-stakeholder-dialogue-and-involvement.html
Author byline as per print journal: Sabine Vogler, PhD; Nina Zimmermann, MA
|
Introduction: This Conference Report aims to provide an overview of key results, messages and conclusions of the Pharmaceutical Pricing and Reimbursement Information (PPRI) Conference with regard to generics. |
Submitted: 22 May 2012; Revised: 5 August 2012; Accepted: 10 August 2012; Published online first: 31 August 2012
On 29 and 30 September 2011, the World Health Organization (WHO) Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies organized the second PPRI Conference in Vienna, Austria. This was attended by 275 delegates (officials and staff of public authorities and payers, pharmaceutical companies, researchers) from 56 countries, and included 60 speakers, panelists and chairs. It addressed pharmaceutical policies from a public health perspective, from both a European and global context.
The first PPRI Conference took place in June 2007 in order to present preliminary results of the research project ‘Pharmaceutical Pricing and Reimbursement Information (PPRI)’ co-funded by the European Commission, Directorate General Public Health and Consumers (DG SANCO) which ran from September 2005 until the beginning of 2008 [1–3]. The first PPRI Conference focused on European pricing and reimbursement policies [4], but generics policies were not a key topic at the conference. Just one presentation, by Professor Richard Laing from WHO, about availability, affordability and price components of medicines in developing and transitional countries [5], highlighting price differences between originators and generic medicines assessed according to WHO/HAI (Health Action International) methodology [6, 7], addressed the generics policy.
The second PPRI Conference was organized in response to requests from participants at the previous conference for a follow-up, and was an activity under the terms of reference of the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies (established in June 2011). The conference objective was to provide information about the activities and the results of the PPRI and Pharmaceutical Health Information System (PHIS) projects. The PPRI initiative has continued as a voluntary networking and information sharing initiative of competent authorities for pricing and reimbursement after the PPRI project officially ended in 2008. The PHIS project (September 2008 to April 2011) [8] was based on the lessons learned and looked particularly at medicines management in the inpatient sector, for which scant literature existed. As a result, the PHIS project called for an urgent improvement in cooperation at the interface between outpatient and hospital sectors [9].
The second PPRI Conference therefore had a focus on ‘hospital pharma and interface management’ and contained three parallel strands: (1) pricing and reimbursement, (2) rational use of medicines and (3) hospital pharma and interface management. The majority of the participants (more than 50 per cent) attended the strand on ‘pricing and reimbursement’. The topics of generics and generics policies appeared in all three strands.
Generics were mentioned in 48 (64%) of the total of 75 contributions (presentations, posters, panel discussions), and in 22 of 48 accepted abstracts (46%). During the first high-level panel discussion Mr Kees de Joncheere, then Regional Adviser for Health Technology and Pharmaceuticals for WHO Europe (now Head of the Essential Medicines Department in WHO Headquarters), stressed the great potential of generics policies and the need to apply these more frequently. Mr Richard Bergstrom, President of the European Federation of Pharmaceutical Industries and Associations stated that ‘once patents expire, prices should fall to a low, but sustainable, level’. In this panel discussion but also throughout the conference there appeared to be a common understanding that generics competition works well. Dr Andreas Seiter, Senior Health Specialist, World Bank [10], expressed concern about having a pricing regulation policy that fixes a difference between the originator and the generic(s) (so-called ‘generic price link’ [11]) because this could impede further benefits and savings that could be achieved through full generics competition.
Presentations and posters described the pharmaceutical situation in 37 different countries, including generics policies and their outcomes. In Croatia, for instance, a generic price link and reference price system, together with policy measures targeting new medicines, have contributed significant savings to the Croatian Social Insurance in 2009 and 2010. Expenditure by Croatian Social Insurance on medicines in the outpatient sector corresponded to Euros 393 million in 2010, 2.9% lower than in the previous year. And in the first months of 2010, public pharmaceutical expenditure in the outpatient sector was 13% lower than that of the first half year of 2009, resulting in a 22% reduction in the health insurance deficit. The savings enabled 47 new innovative medicines to be added to the positive list, i.e. the list of medicines that may be prescribed at the expense of a public payer [12] and 13 medicines to the list of expensive hospital products from July 2009 to July 2010 [13].
Generics policies were highlighted as a policy option for countries, e.g. Ireland, Portugal, and Spain, that needed to implement a bundle of measures in order to respond to the global financial crisis. The crisis created, as one participant [14] commented, ‘a sense of urgency’ to implementing measures. Dr Miguel Gomes from the Portuguese Medicines Agency [15] reported that following the ‘Memorandum of Understanding’ with the ‘Troika’ (International Monetary Fund, European Central Bank, European Commission), savings measures were swiftly implemented. Previously, Portugal’s pricing system for generics set a reference price, i.e. reimbursement amount within a cluster of medicines of identical active ingredient, dosage and pharmaceutical form [12]; defined by the highest priced generics in the cluster [11, 16]. This situation had been criticized as a lost opportunity for savings [17, 18]. Portugal was the only country of the EU to have higher generics market shares in value than in volume [1], which is an indicator of a high price level of generic medicines. A change in methodology in 2010 led to the reference price being redefined as the average of the five cheapest generics [19]. Dr Gomes presented data on how the generics market had developed well, from a starting point of 3.5% in volume in 2003 to a break-even point in 2010 at which generics shares became lower in value than in volume, see Figure 1. Additional data on the development of the average generics prices confirmed a reduction in prices of generic medicines from the first months of 2010 onwards [15]. Generics policies therefore need to be designed for maximum potential benefit.
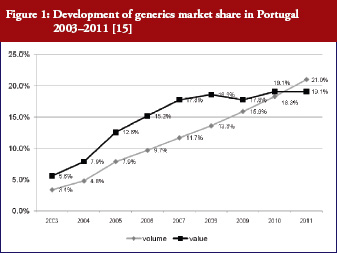 [15]
[15]
Some European countries reported about differences in price between original products and generic medicines, for example, regarding generic omeprazole and simvastatin [20], see also the article [21] generic ACE (angiotensin-converting-enzyme) inhibitors and SSRI’s (selective serotonin re-uptake inhibitors). Dr Kristina Garuoliene, from the Lithuanian National Health Insurance Fund demonstrated how her country, with its small population, was able to achieve considerable price reductions for generic versus originator medicines, see Table 1 [22]. Dr Kristina Garuoliene also presented a case study of clopidogrel and described how public authorities and payers were concerned about how differences in the salt composition, and indications, between originator and generic clopidogrel could potentially reduce savings from generics availability. Responding to efforts by the originator company to retain sales, authorities adopted ‘pragmatic approaches’ to enhance generics prescribing, e.g. mandatory generics substitution, educational activities, see Table 2 [23, 24]. Still, the differences in the reimbursed prices of generic clopidogrel versus the originator were high across Europe, especially briefly after launch, but decreasing over time.
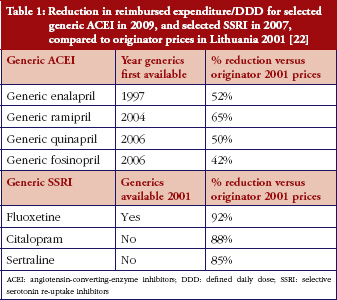 [22]
[22]
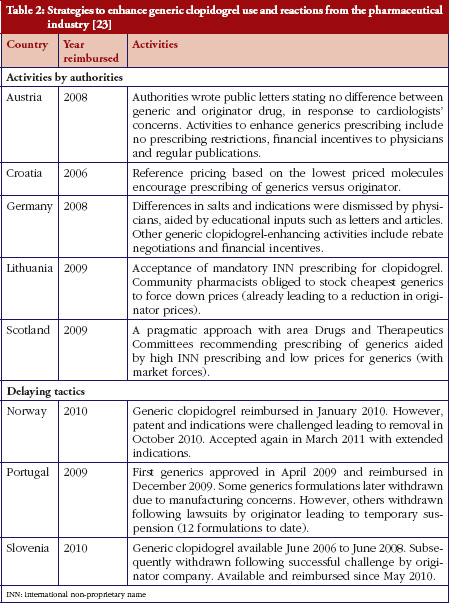 [23]
[23]
In Abu Dhabi, a new policy for generic medicines, including compulsory international non-proprietary name prescribing, was introduced in 2009. However, the expected savings did not fully materialize since there were no accompanying measures to support an increased generics uptake. Abu Dhabi is now exploring a policy of introducing demand-side measures, including educational activities and the introduction of a reference price system for medicines grouped together if they had the same active ingredients [25].
The relevance of generics was highlighted at the fringe ‘Meet the Editors’ session where Dr Brian Godman presented the Generics and Biosimilars Initiative Journal (www.gabi-journal.net), of which he is a member of the International Editorial Advisory Board.
In the final panel discussion, experts from the Norwegian Directorate of Health, OECD, the Southern African Development Community, the WHO Collaborating Centre at Harvard University and WHO Europe stressed the importance of generics policies. The following consensus statement was issued in a conclusion document: ‘Generic[s] policies appear not to be fully exploited yet. At the PPRI Conference, generics were identified as one area where competition works. There is common understanding that savings from generics might be invested for funding innovation. However, as evidence on generics penetration across the countries demonstrated, generics uptake could be improved by more consistent generic[s] policies’ [26]. The PPRI Conference confirmed that in order to achieve the best possible benefits from generics policies, they have to be carefully designed and implemented in a consistent way.
This is an original report from the PPRI Conference. Conference presentations, abstracts and posters are publicly available from: http://whocc.goeg.at/Conference2011/Programme
The PPRI Conference offered several good practice examples as well as a few less successful stories about the implementation of generics policies. Such sharing of information is crucial for policymakers to know the impact of generics policies. Well-informed policymakers can contribute more effectively towards improving the accessibility of medicines, e.g. by applying generics policies in a consistent way and thus creating opportunities for innovation.
A further conclusion from the conference was that the perspectives of consumers and patients should be taken into account. Participants were reminded that pharmaceutical policies should benefit all citizens, especially vulnerable groups, and that their perspectives should be actively considered.
We are very thankful to all who contributed to the success of the PPRI Conference. First of all, we thank the scientific programme committee who supported the organizers in the development of the agenda including the selection of abstracts. Members of the scientific programme committee of the PPRI Conference included Mr Jaime Espin Balbino from the Andalusian School of Public Health (EASP); Ms Margaret Ewen from Health Action International (HAI); Mr Kees de Joncheere, WHO; Professor Richard Laing, WHO; Ms Aukje Mantel-Teeuwisse, Utrecht University, WHO Collaborating Centre for Pharmacoepidemiology and Pharmaceutical Policy Analysis; Mr Øyvind Melien, Norwegian Directorate of Health; Ms Claudia Habl from the Austrian Health Institute (GÖG); and the authors of this manuscript. Further, we thank all speakers, panelists and chairs for their contributions. We particularly thank all those who submitted a large number of high quality abstracts, and those who actively participated with questions and comments. Finally, we are grateful to colleagues in the Austrian Health Institute for their organisational help.
The PPRI Conference was organized by the WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, located at the Health Economics Department of Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG – Austrian Health Institute under its Terms of Reference as a WHO Collaborating Centre (//apps.who.int/whocc/Detail.aspx?cc_ref=AUT-14&cc_city=vienna&). Funding for the Vienna WHO Collaborating Centre’s activities is provided for by the Austrian Federal Ministry of Health who is the legal owner of the Austrian Health Institute. The PPRI Conference was open to anybody interested in the field. Participants were charged a conference fee to cover logistical and organizational costs.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
Nina Zimmermann, MA, Researcher, Geschäftsbereich ÖBIG, Austria
References
1. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. PPRI Report. Vienna: Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG; 2008.
2. Vogler S, Espin J, Habl C. Pharmaceutical Pricing and Reimbursement Information (PPRI) – New PPRI analysis including Spain. Pharmaceuticals Policy and Law. 2009;11(3):213-34.
3. Arts D, Habl C, Rosian I, Vogler S. Pharmaceutical Pricing and Reimbursement Information (PPRI): a European Union project. Italian Journal of Public Health. 2006;3(1):36-40.
4. Vogler S, Habl C, Leopold C, Rosian-Schikuta I. Agenda. PPRI Conference; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2007.
5. Laing R. Pharmaceutical Pricing – Availability, Affordability and Price Components in developing and transitional countries. PPRI Conference; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2007. Available from: http://ppri.goeg.at/Downloads/Presentations/05_WHO_Geneve_Mr_Laing.pdf
6. Gelders S, Ewen M, Nogucchi N, Laing R. Price, availability and affordability. An international comparison of chronic disease medicines. Background report prepared for the WHO Planning Meeting on the Global Initiative for Treatment of Chronic Diseases, Cairo, December 2005. Buch, Monographie. Cairo: World Health Organization, Regional Office for the Eastern Mediterranean, 2006.
7. WHO, Health Action International (HAI). Medicine prices: a new approach to measurement. Working draft for field testing and revision. 2003.
8. Hoebert J, Mantel-Teuwisse A. PHIS (Pharmaceutical Health Information System) Evaluation Report. Utrecht: Utrecht WHO Collaborating Centre for Pharmacoepidemiology and Pharmaceutical Policy, 2011.
9. Vogler S, Habl C, Leopold C, Mazag J, Morak S, Zimmermann N. PHIS Hospital Pharma Report. Vienna: Pharmaceutical Health Information System; Gesundheit Österreich GmbH / Geschäftsbereich ÖBIG; 2010.
10. Seiter A. Pricing and reimbursement of medicines from global perspective. PPRI Conference. Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/PraesentationenPPRIKonferenz/Day2_afternoon_Festsaal_1400_Seiter.pdf
11. Vogler S. The impact of pharmaceutical pricing and reimbursement policies on generics uptake: implementation of policy options on generics in 29 European countries – an overview. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):93-100. doi:10.5639/gabij.2012.0102.020
12. WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies [homepage on the Internet]. Glossary of pharmaceutical terms. Vienna: 2011 [latest update of print version: 2011; regularly updated online; cited 2012 Aug 31]. Available from: http://whocc.goeg.at/Glossary/Search
13. Voncina L, Strizrep T, Godman B, Vlahovi-Palevski V. Recent policies to reduce drug costs and the budget deficit in Croatia: impact and example to others. PPRI Conference Abstract Book; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/Abstract_poster_book.pdf
14. Koopmanschap M. Generic medicine pricing: on track in Europe? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):5. doi:10.5639/gabij.2012.0101.002
15. Gomes M. Pricing and reimbursement policies in the light of the financial crisis. Country examples: Portugal. PPRI Conference. Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/PraesentationenPPRIKonferenz/Day1_afternoon_Festsaal_1300_Gomes.pdf
16. Teixeira I, Vieira I. PPRI Pharma Profile. Portugal. Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2008.
17. Habl C, Vogler S, Leopold C, Schmickl B, Fröschl B. Referenzpreissysteme in Europa. Analyse und Umsetzungsvoraussetzungen für Österreich. Wien: ÖBIG Forschungs- und Planungsgesellschaft mbH; 2008.
18. Vogler S, Leopold C. Access to essential medicines in Portugal. Vienna: ÖBIG Forschungs- und Planungsgesellschaft mbH; 2009.
19. Vogler S, Zimmermann N, Leopold C, de Joncheere K. Pharmaceutical policies in European countries in response to the global financial crisis. Southern Med Review. 2011;4(2):22-32.
20. Godman B, Burkhardt T, Garuoliene K, Teixeira I, Tulunay FC, Gustafsson L. Trends in generic pricing policies in Europe: implications for sustaining equitable and comprehensive healthcare. PPRI Conference Abstract Book; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/Abstract_poster_book.pdf
21. Godman B, Wettermark B, Bishop I. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
22. Garuoliene K, Gulbinovi J, Godman B, Wettermark B, Haycox A. European countries with small populations cannot obtain appreciable price reductions for generics: Lithuania – case history to contradict this. PPRI Conference Abstract Book; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/Abstract_poster_book.pdf
23. Baumgärtel C, Garuoliene K. Enhancing the utilisation of generic clopidogrel: a case history for future guidance? PPRI Conference. Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/PraesentationenPPRIKonferenz/Day2_morning_Sitzungssaal_1130_Baumgärtel.pdf
24. Baumgärtel C, Godman B, Malmstrom R. What lessons can be learned from the launch of generic clopidogrel? Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68. doi:10.5639/gabij.2012.0102.016
25. Abuelkhair M, Godman B, Fahmy S, Abdu S, Gustafsson LL. Challenges when introducing policies to engineer low prices for generics: experiences from Abu Dhabi. PPRI Conference Abstract Book; Vienna: PPRI (Pharmaceutical Pricing and Reimbursement Information); 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/Abstract_poster_book.pdf
26. WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies. PPRI Conference. Final conclusions. Vienna: 2011. Available from: http://whocc.goeg.at/Downloads/Conference2011/PPRI_Conference_Conclusions_final.pdf
|
Author for correspondence: Sabine Vogler, PhD, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Health Economics Department, Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG – Austrian Health Institute, 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Related article
Generics policies–a globally-relevant implementation challenge
Source URL: https://gabi-journal.net/the-potential-of-generics-policies-more-room-for-exploitation-ppri-conference-report.html
|
Introduction: To describe pharmaceutical pricing and reimbursement systems in 29 European countries with regard to medicines, particularly generics, and their possible impact on generics uptake. |
Submitted: 18 April 2011; Revised manuscript received: 5 March 2012; Accepted: 9 March 2012
This article aims to provide an overview about pharmaceutical pricing and reimbursement policies which European countries, including all EU Member States, apply to promote the use of generic medicines.
In the EU, Member States are free to develop their national– and regional–pharmaceutical policies in the field of pricing and reimbursement. This is permitted so long as they comply with overall EU provisions, such as the Transparency Directive [1], e.g. formal procedural aspects such as deadlines or a transparent publication policy. Despite EU harmonisation of marketing authorisation [2], EU Member States can freely design their pharmaceutical pricing and reimbursement framework, thus opting for more market control and/or promoting competition with regard to generics and other pharmaceutical products.
All European countries are facing growing pharmaceutical expenditure, particularly in public expenditure [3] given that Europe has a comparatively higher coverage of publicly funded health care compared to the rest of the world [4]. For example, two thirds of European pharmaceutical expenditure is funded by a third party payer such as social health insurance, despite individual countries varying in the share of public funded medicines [5, 6]. Public pharmaceutical expenditure in the outpatient sector increased in EU countries by 76% between 2000 and 2009 (median: 53%; lowest value: 21%; highest value: 243%) [5, 7]. Aggravating the situation further, the global financial crisis has forced several European countries to introduce short-term rigid cost-containment measures [8].
With limited budgets, European countries view generics as a policy option that enables savings to be made and which can then be used for funding high-cost medicines. As stated in the European Commission’s Communication on a Renewed Vision of the Pharmaceutical Sector: ‘[m]any Member States recognise that generic medicines play an important role in helping to limit their healthcare expenditure in their reimbursement and prescribing practices. Competition with off-patent products enables sustainable treatment of more patients with less financial resources. The generated savings create financial headroom for innovative medicines’ [9].
Different European countries vary in the size of the pharmaceutical market occupied by generic medicines, with high shares apparent for several central and eastern European countries, Germany, The Netherlands and UK. Figure 1 also shows that some countries, while starting at a lower point, have succeeded in growing their generics markets in recent years.
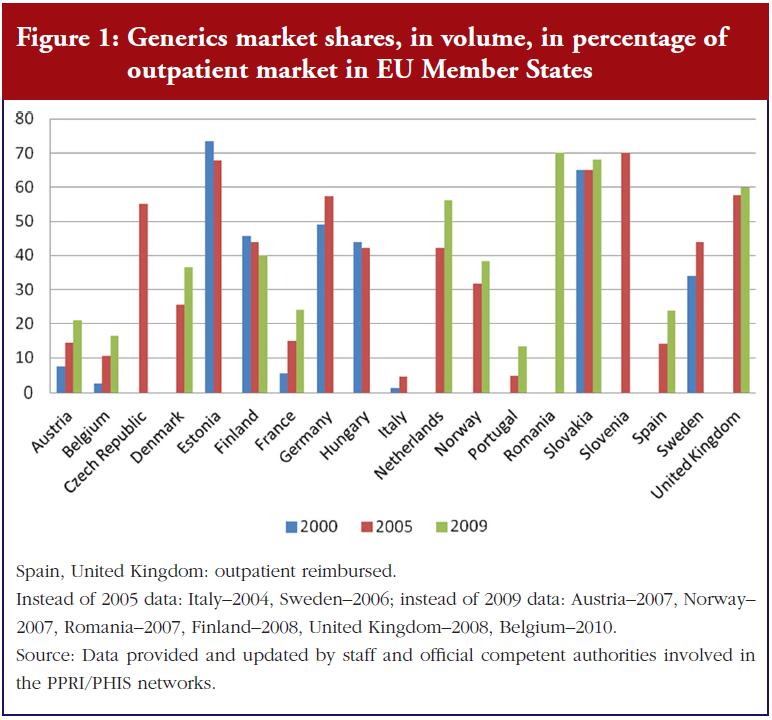
This article provides: first, an up-to-date picture of the pharmaceutical pricing and reimbursement policies aimed at promoting generics uptake, and second, a discussion of the possible impact of different generics policies on the generics market.
The author and her team collected information from European countries on generics policies and further measures that have an impact on generics use. This was achieved through direct contact with representatives of the competent public authorities responsible for pharmaceutical pricing and reimbursement.
The author and her team have established good contacts with national officials in their role as coordinators of the Pharmaceutical Pricing and Reimbursement Information (PPRI) and the Pharmaceutical Health Information System (PHIS) networks, to which representatives of public authorities belong. PPRI is a networking and information-sharing initiative on pharmaceutical policies which emerged from a European Commission co-funded project under the same name [6, 10]. As of February 2012, PPRI consisted of more than 60 institutions, mainly medicines agencies, ministries of health, and social insurance institutions, from 39 countries. These include all 27 EU Member States, nine further European countries and three non-European countries, plus European and international institutions (European Commission services and agencies, OECD, WHO and World Bank). PHIS was a similar project, broader in scope (including the hospital sector), and involving both public authority staff and hospital pharmacists [11].
These two projects produced a wealth of information which was considered when designing and drafting this article. Of particular relevance were the PPRI and PHIS Pharma Profiles–national country reports about the pharmaceutical pricing and reimbursement system, written by public authority staff.
After identifying relevant information and policies, data were collected in three steps in the spring of 2011:
The article focuses on EU. It covers all 27 EU Member States plus the candidate countries Croatia and Norway–for its interesting generics policies. The manuscript was revised according to changes reported by PPRI members during 2011 and in early 2012.
The pricing and reimbursement framework is an essential part of a pharmaceutical system. Pharmaceutical pricing and reimbursement policies might be individually designed for different groups of medicines such as innovative medicines, hospital medicines and/or generics.
In terms of pricing, a country may allow free pricing for (some) medicines, i.e. the manufacturer may freely decide on the price, or the authorities can regulate medicine prices according to various price setting methodologies and criteria.
In most European countries manufacturer prices (ex-factory prices) are directly regulated by the State, see Table 1. However, in a few countries (Cyprus–for imported medicines, Denmark, Finland, Latvia, The Netherlands, Norway, Poland, Sweden and UK) the ex-factory price is indirectly regulated. For example, in Nordic countries, the relevant authorities approve a maximum wholesale price; and in the UK, the Pharmaceutical Pricing Regulation Scheme controls the maximum profits of companies.
At the manufacturer level, most countries surveyed control medicine prices only for reimbursable medicines–whose costs are at least partially covered by the national health services or social health insurance [15]. Five countries apply price regulation to all medicines, whereas Denmark and Germany are typically defined as ‘free pricing countries’. However, even these countries have long-operated price controls on reimbursable off-patent products. Additionally, ongoing reform in Germany has introduced a kind of price control for new medicines [16].
In summary, a major feature of price control is reimbursement status: reimbursable medicines tend to be subject to state price control whereas non-reimbursable medicines are allowed free pricing. The same applies to reimbursable versus non-reimbursable generics.
At the distribution stages (wholesale and pharmacy), the scope of medicines price control is broader: several countries regulate the remuneration for distributors, usually in the form of maximum linear, or regressive, markups. A few other countries only regulate the distribution markup of reimbursable medicines; including generics, see Table 1.
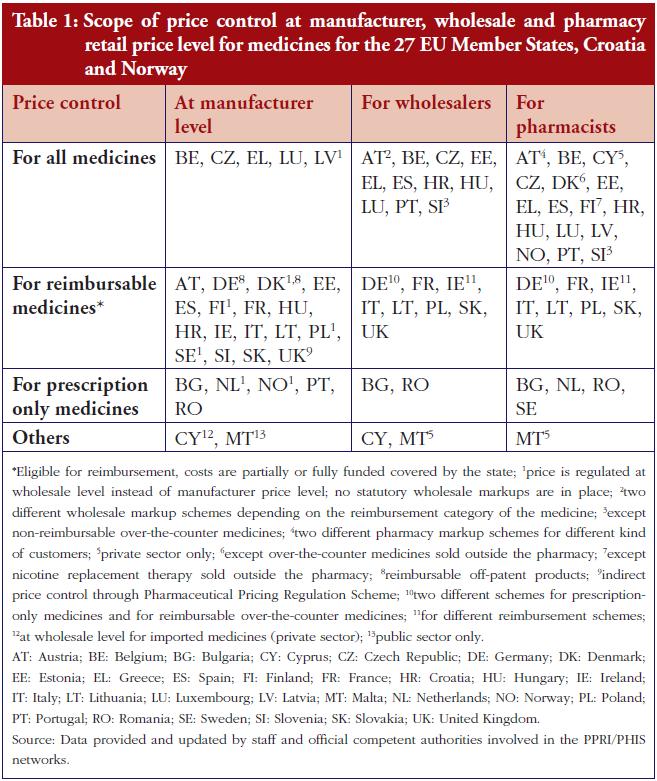
A common method for determining medicine prices is external price referencing–international price comparison. In 25 of the 29 countries surveyed, competent authorities check medicines prices in other countries when setting and/or negotiating a price. Only Denmark, Sweden and UK do not apply external price referencing, while Germany is just beginning to consider medicine prices elsewhere with regard to certain new medicines [7, 12, 13, 16, 17].
External price referencing is typically limited, however, to original products [17]. The most common pricing procedure applied to generic medicines is internal price referencing. This is the practice of using the price(s) of identical or similar products in a country when setting a price.
Sixteen countries surveyed required generics–and other ‘follower products’–to be priced at a certain percentage lower than the originators–a policy called ‘generic price linkage’ [3], see Table 2. Austria and Estonia, for example, specify that not only the first ‘follower’, but also all additional followers and the original products are required to lower their price. Since 2005, Norway has used the ‘stepped price model’ (Trinnprismodellen) to incrementally reduce the price of a medicine according to predefined rates, depending on sales volumes.
The first reduction occurs after a medicine has lost patent protection [21, 22].
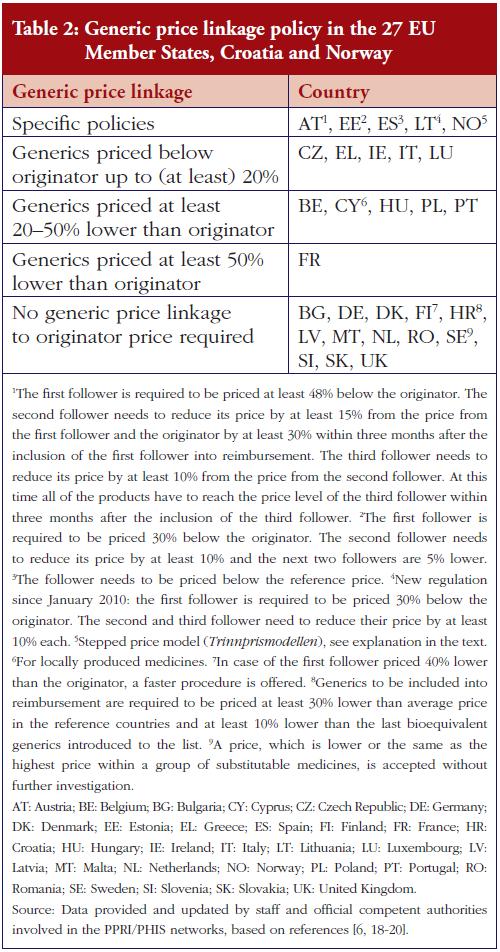
Key elements of pharmaceutical reimbursement systems are reference price systems and reimbursement lists– positive lists for medicines eligible for reimbursement and negative lists specifying products explicitly excluded from reimbursement [15].
Positive lists are in place in 26 of the surveyed countries (all but Germany, Spain, UK), whereas negative lists are only used in Germany, Hungary, Spain, and UK. Thirdparty payers do not automatically cover the full costs of medicines from the positive lists. Full reimbursement of eligible medicines only occurs in seven countries– Austria, Germany, Ireland, Italy, Malta, The Netherlands, and UK, while elsewhere reimbursement for several medicines is at a lower rate. Criteria for inclusion on a positive list include cost-effectiveness, medical need and therapeutic value–in particular, the relative effectiveness in the case of medicines with no new, but added therapeutic value. Another consideration is the estimated budget impact–in central and eastern European countries [7].
A reference price system is a major policy option for the promotion of generics uptake. This entails clustering identical or similar products into so-called reference groups. Each cluster has a maximum reimbursement amount (reference price) to be covered by the third-party payer. The patient must pay the difference between this reference price and the actual pharmacy retail price, in addition to any other co-payments [15]. Twenty-two of the countries surveyed have a reference price system, 13 of which specify clusters containing medicines with the same active ingredient, see Figure 2. Most countries define the reference prices around or below the average price of generics, or at the lowest price in the cluster [7, 18, 23].
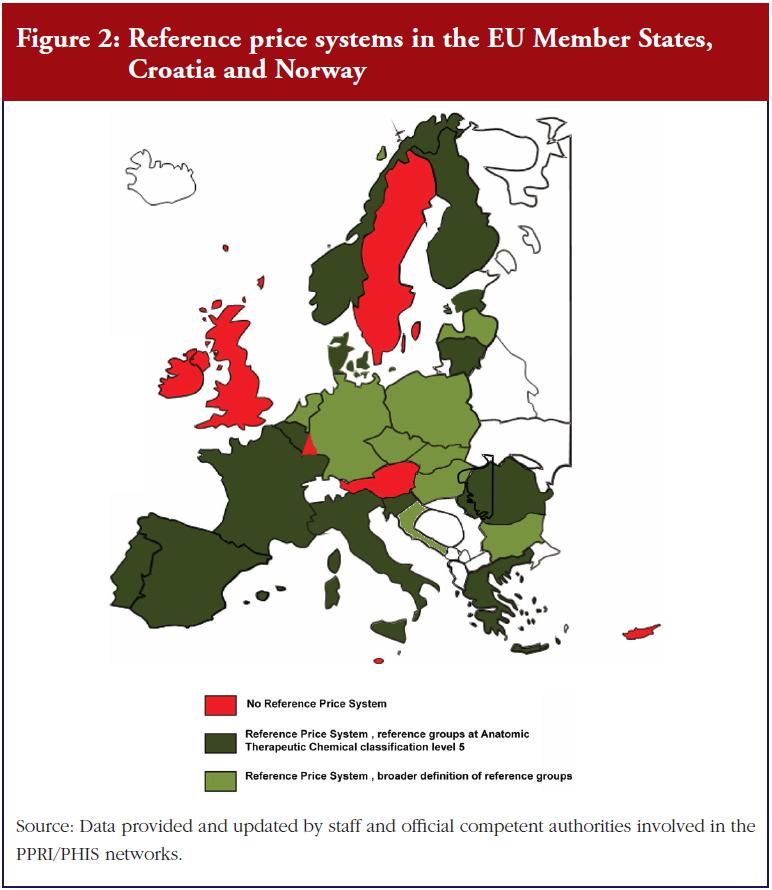
In addition to these pricing and reimbursement policies, European countries also employ specific demand-side measures to promote the use of generics, targeting prescribers, pharmacists and the public. For example, 23 countries surveyed use generics substitution (mandatory in six countries); while 24 countries use international non-proprietary name (INN) prescribing (mandatory in five countries), see Table 3. Public information campaigns often focus on generics with the goal of raising awareness and building trust and/or explaining a specific generics policy [24].
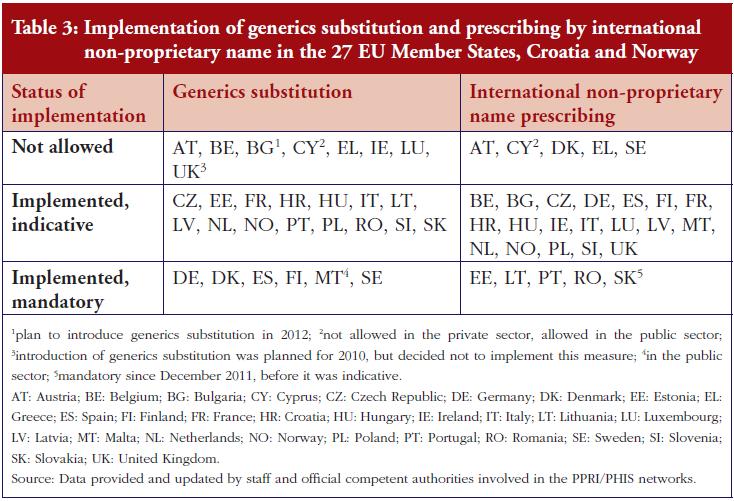
This survey of generics policies and pharmaceutical pricing and reimbursement shows that European pharmaceutical systems have a strong linkage between pricing and reimbursement, and that some policies are only relevant for reimbursable medicines. As a result, only those generics that are eligible for reimbursement are covered by specific regulations such as price control. In contrast, non-reimbursable generics do not fall within the scope of price control. In addition, generics are specifically targeted by policies aimed at enhancing their use and saving money–for public payers. Table 4 gives a country-specific overview of key policies.
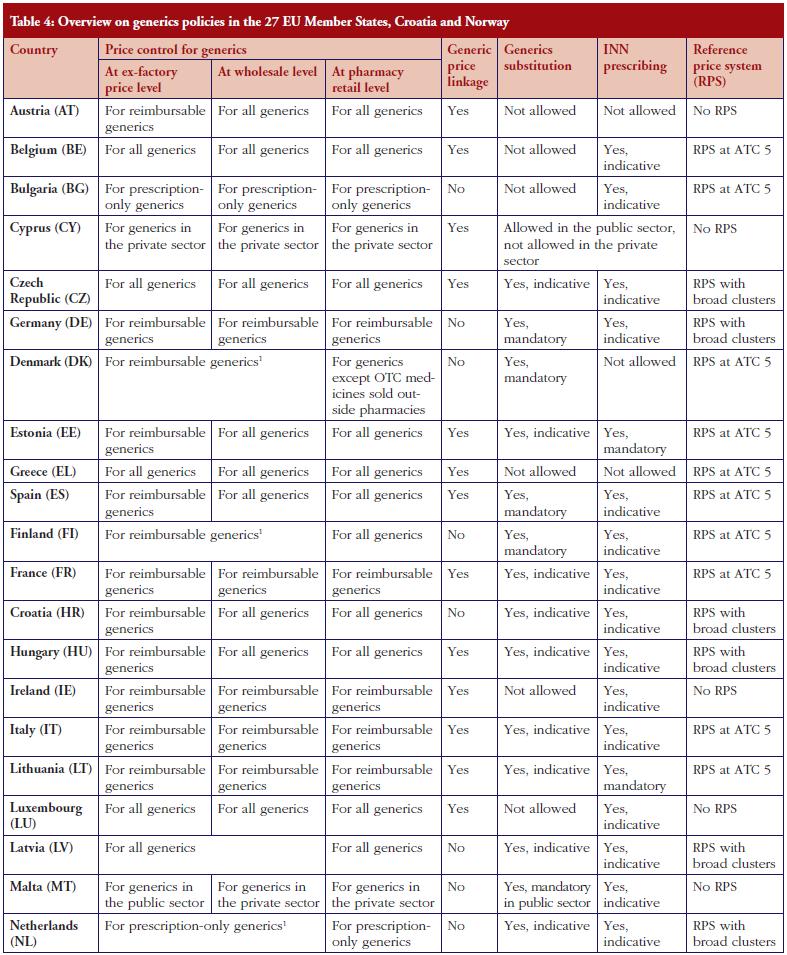
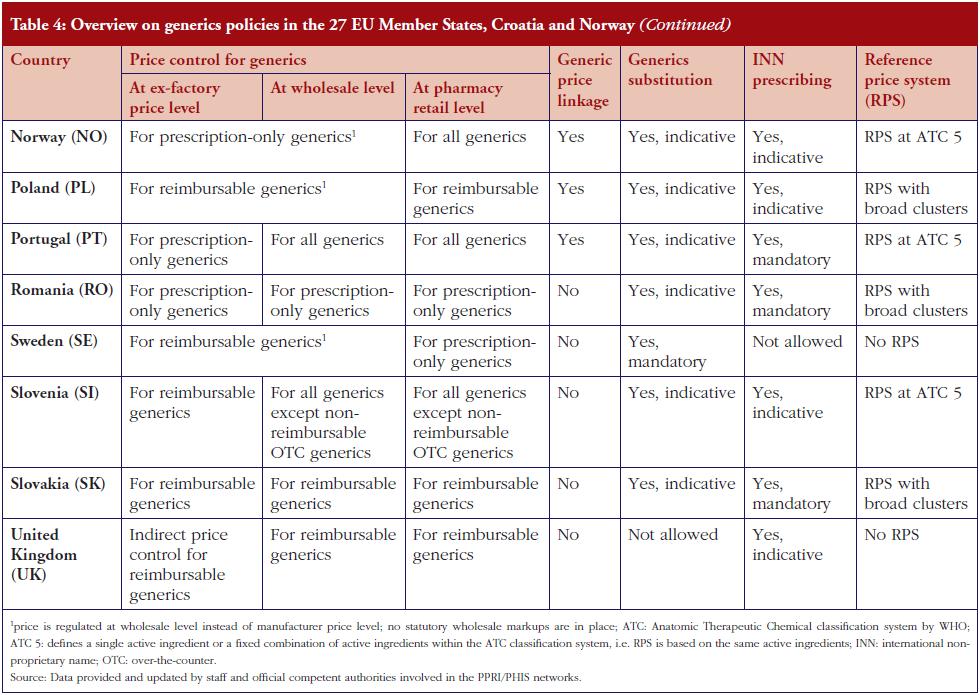
The potential for cost-containment can be seen in the prices of generics which may be set at a lower level (generic price linkage), and via competition from the arrival of new generics on the market. The latter also leads to lower prices of other generics and the originator. Both strategies, partially in parallel, occur in the countries surveyed, and add to a growing body of reports about different generics pricing policies including mixed approaches [25].
Policymakers tend to take a broad view of generics. Several countries promote the use of not just generics but also ‘nonexpensive’ products. These may include copyproducts, parallel imported medicines, for example, in Denmark or Germany [18], and even less expensive original products (as in Belgium [26]). Countries vary as to the necessary price difference between a generic medicine, or other follower products, and the originator. Questions may arise over whether higher price differences are possible, and by how much, and whether lower prices might be achieved for generics through policies other than the generic price linkage. Countries, which do not explicitly require generics to be priced at a certain percentage lower still report considerable price differences, for example, The Netherlands [18, 28] and Slovakia [27]. This they attribute to market competition, for instance, supported by tendering mechanisms in the outpatient sector [29]. In addition, according to a literature review, while generic price linkages and price regulation did succeed in lowering generic medicine prices to a certain point, there were indications for a higher potential for savings which might be triggered by free-price competition in the generics market [30].
A widely-used approach to promote generics in European countries is the introduction of demand-side measures, notably generics substitution and INN prescribing. Ten countries in the survey combined these two measures. A few countries have either one or other in place (six countries with INN prescribing but no generics substitution, three countries with generics substitution but no INN prescribing, see Tables 3 and 4. The choice of just one of the policies could be due to its appearing sufficient for achieving the expected results. In the UK, with a high rate of INN prescribing and a comparatively high generics market share, the government decided against the introduction of generics substitution planned for 2010 following a public consultation [31]).
A common combination, however, is generics substitution or INN prescribing together with a reference price system–seen in 20 countries. In combination, these two tools appear to positively influence each other [6]. Among the countries surveyed, only one country (Austria) lacks all three of the aforementioned policies. Its generics market shares are comparably low, but are now on the increase following the introduction of a generics price linkage policy [32].
It is important to highlight not only the quantity of measures, but also the quality of their implementation. Stringent implementation of a few measures can produce the desired effects. For instance, Sweden produced a high generics market share even after abolishing the reference price system, perhaps because another system of clustering similar medicines already existed, as well as mandatory generics substitution [6, 33, 34] and discussion/references below. Norway’s stepped price model, introduced in 2005, has increased the market share for generics from 31.8% of the volume of the outpatient market in 2005 to 38.4% in 2007, see Figure 1. Norway has thus ‘ensured that prices for generics have fallen’ [22]. But in the context of Norway’s overall pricing policies, the introduction of a pricing regime linked to prices in other European countries, and the design of their external price referencing, had a moderating effect on the Norwegian price level in general [35, 36].
The level of enforcement also affects the success of new measures to promote generics use and control prices [6, 18]. Policy measures, such as generics substitution and/or INN prescribing, might be implemented on a voluntary or a mandatory basis. A voluntary implementation occurs in the majority of countries surveyed, but some, for example, Lithuania and Slovakia, have changed their legislation and made INN prescribing obligatory. This mandatory implementation of a policy has proved successful at increasing generics uptake, for example, in Sweden [6, 37, 38].
Besides mandatory enforcement (possibly accompanied by financial sanctions), incentives for stakeholders can also be an issue. In The Netherlands, a financial incentive was in place for years allowing pharmacists to retain one third of the difference between the price of the medicine and the reference price if they dispensed a medicine below the reference price [39]. This incentive appears to have contributed to an increase in the generics market share, but interestingly, after the abolition of the measure in 2005, the generics substitution rate continued to stay at a high level and even rose (42% in volume of total market in 2005, 56% in 2009, see Figure 1). Some experts feel that the continuing high substitution rate is attributable to a positive attitude that has developed among Dutch pharmacists [18]. Another example is a pilot project in Austria, in which patients of a small sickness fund were charged a lower prescription fee if they obtained a generic instead of a brand-name medication. As a result, the patients asked for the generics and the share of generics prescriptions rose [40].
These examples suggest that in addition to formal mechanisms, such as punishing sanctions or encouraging incentives, a ‘generics culture’, i.e. a positive attitude and trust in generics (and biosimilars), may contribute significantly to an increased use of generics. We recommend the development of such a culture, using strategies that target all stakeholders, including patients.
Finally, the organisation of a reference price system is another trigger for generics uptake. Twenty-two of the European countries surveyed have a reference price system; thirteen of these organise the reference groups at the Anatomic Therapeutic Chemical classification level 5, in other words, clustering of medicines with the same active ingredient, see Figure 2. This is the easiest way to manage the system and probably an appropriate starting point when introducing such a policy. However, care should be taken over the definition of a cluster: a very narrow cluster can result in some patients re-allocating their demand away from the reference group to an alternative non-patent medicine, with the consequent loss of potential savings [18, 23, 39, 41]. Such ‘re-allocation of demand’ has been observed, to some extent, in a few European countries, for example, France and Italy [42].
Another relevant parameter in the design of a reference price system is the fixed reimbursement amount, i.e. the so-called reference price. In mature high-volume generics markets such as in Poland, a higher reimbursement amount might be advisable for a brief period, to provide incentives for generics manufacturers to enter the market [43]. A lower reference price generally results in higher savings. In this respect, Portugal, which initially set the reference price at that of the highest priced generics in the reference group [44, 45], decided in 2010 to reset the price to the average of the five lowest priced medicines [8]. In future it will be useful to examine the impact of this decision on the generics market shares. Further, in five other European countries: Belgium, Estonia, Latvia, Lithuania, Spain; the methodology regarding the calculation of the reference price was changed in 2010 and 2011 [8], apparently for cost-containment reasons, because these countries were strongly hit by the global financial crisis.
European pharmaceutical systems use several different types of pricing and reimbursement policies for medicines including reimbursable medicines. Generics, if deemed reimbursable, are subject to the same policies. In addition, many countries have implemented specific measures to promote generics uptake, including demand-side measures targeting prescribers, pharmacists and, less frequently, patients. Usually, a mix of policies is employed. The design of these measures can significantly influence generics uptake and the degree of public savings. However, the difficulties in enforcing these measures should be addressed. Creating a ‘generics culture’, i.e. an environment which is positive towards generics, appears to support other policy measures.
Several European countries aim to increase generics uptake, this allows offering medicines at lower prices for the sake of patients who have to pay out-of-pocket and/or to co-pay. In the case of publicly funded medicines it also offers savings for the healthcare system and thus provides financial headroom for funding innovation, therefore, increasing both accessibility and affordability to patients. Evidence about generics policies helps not only the policymakers to reach their aims but also the patients
benefiting from improved accessibility and affordability.
The author wishes to express sincere appreciation to the participants of the PPRI/PHIS network members who, by writing country reports and responding to requests for validating the accuracy and timeliness of data, helped to provide information about major pharmaceutical pricing and reimbursement policies. Particular thanks go to the PPRI/PHIS team members at Gesundheit Österreich, Ms Christine Leopold and Ms Nina Zimmermann, for their support in surveying and monitoring the information, and Mr Borja Garcia-Lorenzo, intern at Gesundheit Österreich, for his assistance with the figures used in this article.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
|
Author for correspondence: Sabine Vogler, PhD, Health Economics Department, Head of WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies, Gesundheit Österreich (Austrian Health Institute)/Geschäftsbereich ÖBIG, 6 Stubenring, AT-1010 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Related articles
Payers endorse generics to enhance prescribing efficiency: impact and future implications, a case history approach
A review of generic medicine pricing in Europe
European payer initiatives to reduce prescribing costs through use of generics
Source URL: https://gabi-journal.net/the-impact-of-pharmaceutical-pricing-and-reimbursement-policies-on-generics-uptake-implementation-of-policy-options-on-generics-in-29-european-countries%e2%94%80an-overview.html
Copyright ©2024 GaBI Journal unless otherwise noted.