Author byline as per print journal: Auxiliary Professor Antistio Alviz-Amador1, MSc, Pharmaceutical Chemist; Jairo E Mercado Camargo2, MSc; Marlene Duran-Lengua3, MSc, PhD, Bacteriologist
|
Introduction: Moxifloxacin is a quinolone antibiotic often used to treat infections caused by Gram-positive bacteria that have developed resistance in Colombia in recent years. |
Submitted: 1 May 2018; Revised: 6 September 2018; Accepted: 2 October 2018; Published online first: 15 October 2018
Introduction
The number and variety of medicinal products available to patients and the pharmaceutical market is constantly evolving. To ensure the most effective use of medications, an ongoing multidisciplinary evaluation and selection process is needed to guarantee efficacy, safety and affordability [1]. Many new medicines are generics and a generic is defined as ‘A drug product that has the same composition in active substance(s) and the same pharmaceutical form as the originator reference medicine, as well as the same dosage form, strength, route of administration, quality and performance characteristics and intended use, and whose bioequivalence with the originator reference medicine, i.e. the same behaviour in the body, has been demonstrated by appropriate bioequivalence studies’ [2]. Generics cost less than innovator products as they are marketed after the innovator patent has expired and they do not require the same level of investment and clinical studies necessary when developing innovative chemical entities [3]. Generic medications are a viable economic alternative to help reduce the burden on healthcare budgets in developed nations and to facilitate access to treatment in developing nations [4]. However, worldwide controversy and debate has cast doubt on their efficacy, causing a degree of uncertainty among healthcare professionals, the pharmaceutical industry, consumer and governmental agencies [5].
Despite concerns, the World Health Organization (WHO) and Drug Regulation Affairs (DRA) agencies support generics commercialization because generics facilitate cost control and are an irreplaceable therapeutic alternative in countries that do not have access to the original formulation [6]. As such, the debate surrounding the use of generics continues around the world. Their efficacy and reliability is often challenged but must be guaranteed to ensure that patient health is not jeopardized [7]. In addition, it seems that belief in ‘the greater the cost, the greater efficiency’ has generated a lack of public confidence in generic medications. This has created a false perception that they are weaker, lower quality, unreliable products that put patient health at risk [8].
In Colombia, the high cost of innovator medicinal products has reduced their usage in recent years. They are being replaced by homologous generics and in November 2010, the Colombian Ministry of Health and Social Protection (Ministerio de Salud y Protección Social, MinSalud) issued Government Resolution Number 4377, which ruled that all patient’s prescription medications should be generics as the price difference has been proven to allow for huge national savings [8].
With respect to antibiotics, generic antibiotic’s low cost has meant that they have been used irrationally which has caused many common pathogens to have high levels of antibiotic resistance [9]. Within the large arsenal of available antibiotics, broad resistance to quinolones has been reported, especially the third and fourth generation such as moxifloxacin [10] as these are widely used in clinical medicine to combat infection caused by Gram positive bacteria.
Comparative studies of the in vitro interchangeability of alternative pharmaceuticals (generic versus brand-name drug) of moxifloxacin have not been undertaken and, in Colombia, this led to reduced public confidence in generic versions of this antibiotic. As such, there was an obvious need to undertake studies to evaluate both the generic and brand-name pharmaceutical alternatives of moxifloxacin and their effectiveness against resistant and sensitive strains of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) isolated from hospitalized patients [7].
This study was designed to evaluate the concentration of the main active ingredient in moxifloxacin drug products by high performance liquid chromatography (HPLC); together with the antibacterial activity, which was understood by determining the minimum inhibitory concentration (MIC) of two generic versions versus United States Pharmacopeia (USP) standards of moxifloxacin against strains of S. aureus and E. coli isolated from hospitalized patients. These are American Type Culture Collection (ATCC) strains that are both sensitive and resistant.
Methods
Materials and equipment
Three batches of moxifloxacin were obtained. The generic versions were produced by Pharmedic and ADS Pharma, and the USP standard was made by Sigma Aldrich (Current Lot F009J0). The culture media used for the growth of the microorganisms were: Nutritious Agar (Merck Millipore), Trypticase Soy Agar (Merck Millipore), and liquid media which included: Thioglycolate Broth (Merck Millipore), Muller-Hinton Broth (Merck Millipore), and LB broth (Merck Millipore).
Chromatography
HPLC was performed on a Hitachi Elite LaChrom chromatographic system, equipped with a quaternary pump (L-2130), Autosampler (L-2200) and a Diode Array Detector (DAD). A reversed phase column LiChrospher® C18, 150 × 4.6 mm, particle size 5 μm (Merck®), was used as the analytical column. The mobile phase was a mixture of 0.1% solution of triethanolamine-acetonitrile (80:20 v/v), adjusted to a pH of 4.8 with phosphoric acid, and filtered and degassed by a 0.45 μm membrane. The wavelength was set at 296 nm, with flow rate of 1 mL/min and injection volume of 20 μL. A chromatographic method was implemented and validated for the determination of moxifloxacin according to what is reported in the USP 38 and is described below [11].
Validation parameters
Linearity
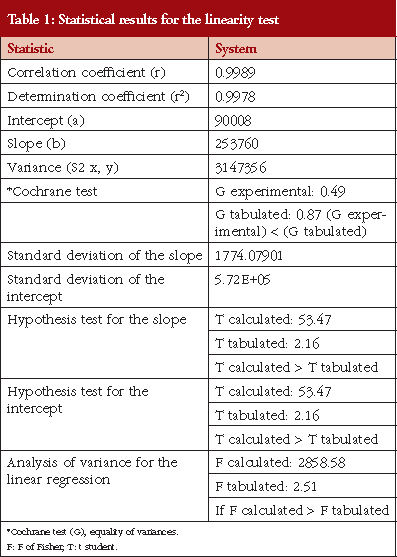
A calibration curve was prepared with five corresponding concentrations of 80%, 90%, 100%, 110% and 120% of the theoretical concentration (nominal concentration 0.1 mg/mL). All concentrations were injected in triplicate. This facilitated linear regression analysis.
Accuracy and precision
The accuracy was determined at three different concentrations of moxifloxacin: low (50 mg/L), medium (75 mg/L) and high (100 mg/L), which were all prepared from a stock solution of 1,000 mg/L. For intermediate precision, two different analysts took the 100 mg/L solution on three different days and the relative standard deviation (RSD) was calculated using an RSD × 2% as the acceptation criterion.
Selectivity
Selectivity was evaluated by preparing a 100 mg/L solution of the working concentration. Three aliquots of this solution were subjected to the following extreme conditions: 0.1 M hydrochloric acid, 30% sodium hydroxide, and reflux for an hour at 80°C, with their respective placebo and standard solutions. Finally, they were injected to HPLC equipment to evaluate the degradation grade of the moxifloxacin.
Antibacterial activity
Microorganisms
The ATCC strains of bacteria used were: S. aureus 43300 Methicillin-resistant (SAMR), S. aureus 25923 Methicillin-sensitive (SAMS), and E. coli 25922 purchased from the authorized Techno medical distributor. Clinical isolations were obtained from two Colombian Hospital laboratories (from ICU). Two new strains of S. luteus canariensis and E. coli tropicali were supplied from MICROKIT Laboratories.
Reactivation of the strains
A lenticule of new wild type Colombian strain E. coli tropicali, S. luteus canariensis, and those bacteria isolated from ICU (S. aureus and E. coli) were dissolved in 4 mL of thioglycolate broth and incubated for 24 hours. 500 μL of the suspension was then added to 4 mL of LB broth, which was incubated for a further 24 hours at 37°C. Then, 800 μL of the latter was put it into a 1 mL Eppendorf and 10% glycerol was added while the solution was kept at –80°C [12].
Ensuring the purity of the inoculate
After reactivation, 5 μL of the SAMR and SAMS, and E. coli bacterial suspensions were cultivated in a thioglycolate broth. Subcultures were then made using a nutrient agar plate. The inoculated plates were incubated for 16 hours at 37°C and examined to ensure that there were no contaminants [13].
The confirmation was carried out based on identification tests of strains used, which included biochemical and growth tests in selective and differential cultures using the MicroScan AutoSCAN-4 kit, in addition to routine Gram staining, for later observation under an optical microscope.
Bacterial growth curve
Four morphologically similar bacterial colonies were inoculated in a beaker of Müller-Hinton Broth and incubated at 37°C. A Multiskan EX Thermos Scientific microplate spectrographic reader (wavelength of 620 nm an hour, range 0 to 48 hours) was used to measure their optical density. Each reading was carried out in triplicate in a 96-well microplate. The Müller-Hinton Broth was used as the sterile control and the average of the absorbance was plotted against time to determine the period of maximum growth [14].
Inoculum preparation
The bacterial strains were scattered on the surface of the agar nutrient (SAMR and SAMS and E. coli) to guarantee their viability.
Inoculating of the bacteria
Three bacterial colonies were selected from the agar plate and inoculated in a 5 mL beaker of Müller-Hinton Broth, which was incubated at 37°C for 2−5 hours until it reached 0.5 turbidity (equivalent to McFarland standard (about 1 × 108 UFC/mL). Where necessary, the turbidity of the broth was adjusted by adding more broth. The resulting suspension was approximately 1 × 108 UFC/mL, which was verified by a Thermo Scientific Multiskan EX®, 620 nm spectrographic reading [13]. A 1/100 dilution of the previous cultivate (100 μL of the bacterial suspension in 9.9 mL of Müller-Hinton Broth) was prepared to obtain a solution of 1 × 106 UFC/mL [15]. Subsequently, this was used to determine the MIC of moxifloxacin for each of the microorganisms in the study.
Determining the minimum inhibitory concentration (MIC)
The two generic (Pharmedic and ADS Pharma) and one USP standard of moxifloxacin were evaluated using the broth micro dilution method, described by the Clinical and Laboratory Standards Institute (CLSI, 2015) [13].
Serial dilutions of each drug were performed from 64 to 0.0075 μg/mL, 50 μL of each antibiotic dilution was dispensed into microplate wells, where 50 μL of the inoculate was added to each one. This produced final bacterial solutions of 5 × 105 UFC/mL, and antibiotic dilutions from 0.015 μg/mL to 8 μg/mL. A well that contained an antibiotic-free inoculate was used as a positive control and one containing only antibiotic without inoculate was used as a negative control. A Thermo Scientific Multiskan EX® spectrophotometer to a wavelength of 620 nm was used to take readings from the microplates [12]. All assays were conducted in triplicate.
Statistical analysis
The growth curve was analysed with descriptive statistics using Microsoft Excel. Inferential statistics were used to test the MIC values obtained from the solutions tested. Specifically, an analysis of variance (ANOVA) was carried out, followed by a Tukey’s test to compare multiples, accepting significant statistical difference to a value of p < 0.05. The Graph Pad Prism program, version 5.01®, was used to plot and tabulate the data as the average ± the standard error of the average.
Results
Finding the validation parameters
Following the methodology described in USP 38 [11] and using the chromatographic conditions described above, the standard moxifloxacin solution was injected three times into the chromatograph so as to check the suitability of the system. This enabled a theoretical number of plates equal to 5282 ± 1.8, with an asymmetry of 1.18 ± 0.9, to be obtained. USP 38 specifies a minimum of 1,200 theoretical plates and an asymmetry of < 2 and as such, we can confirm that the methodology complies with what was anticipated, and the system is ideal.
Linearity
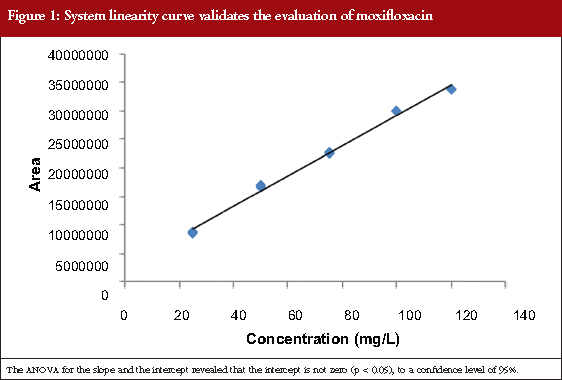
The calibration curve was linear, see Table 1, in the concentration range between 25 and 120 mg/mL (corresponding to 80−110%). Linear regression enables a straight line to be obtained with the equation expressed as Y = 253760X+90008. The linear coefficient correlation (r) and coefficient of determination (r2) were 0.9989 and 0.998, respectively, see Figure 1. This shows that the linearity conditions of the analytical system are satisfied.
Accuracy and precision
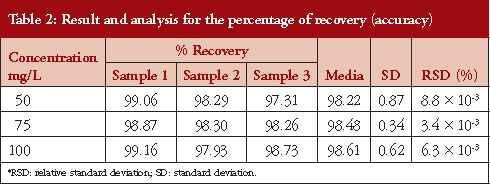
The results related to the accuracy of this study are presented in Table 2. To determine the accuracy, we used the recovery percentage method, which used three concentrations by triplicate: 50, 75 and 100 mg/L. The high recovery rate for moxifloxacin was 98.4% ± 0.61, with an RSD % < 2.
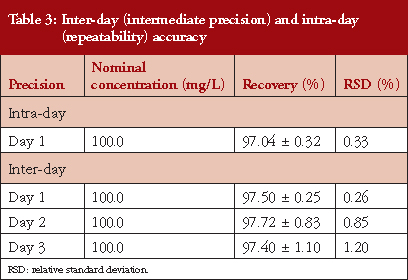
In the specific case of repeatability, a nominal concentration of 100 mg/L was used (carried out in triplicate) and the concentration of each replicate determined, resulting in an average value of (n = 9) 97.04% ± 0.32. The RSD % obtained were < 2%, see Table 3.
Selectivity
In this test, the standard solutions and the sample of generic moxifloxacin were subjected to extreme conditions in acidic, basic and oxidazing media (H2O2), so as to observe whether the analyte caused interference with their generated degradation products. Here, there was a single characteristic peak of moxifloxacin in the chromatogram in a retention time of 4.3 ± 0.06 min in the acid and basic media observed. However, in the oxidation media, a peak product of degradation in the analyte under study was observed to a retention time of 3.0 min, without any interference from the moxifloxacin signal, see chromatograms in the supplementary material. Despite this, the method used here is selective for quantification of the drug of interest.
Microbiology analysis
Bacterial growth
In the supplementary materials for both sensitive and resistant ATCC strains of E. coli and S. aureus, the maximum growth period between 5−20 hours are provided, as is seen in CLSI reports and computational simulations [13, 14]. In addition, the strains of E. coli tropicali and S. luteus canariensis from MICROKIT Laboratories had a maximum growth period that peaked at 24 hours, which corresponds well with those reported by MICROKIT Laboratories in the technical annexes they provided. This indicates that the MICROKIT Laboratories strains showed a maximum growth period that is longer than the ATCC strains.
Microorganisms isolated in different hospitals have been shown to have maximum growth times of 16 hours (S. aureus), and 18 hours (E. coli). They present a time of maximum growth greater than the ATCC strains, and they do not present anomalies with respect to the growth, although the different stages of growth can be observed and differentiated from the assimilation in the medium (< 5 hours) until the stage when bacteria died. In the case of S. aureus, death occurred between 18 and 20 hours and for E. coli it occurred from 19 hours. Comparing these strains with those of ATCC, one can see that maximum growth times of the isolates are greater than the ATCC strains, see Supplementary Materials.
MIC results
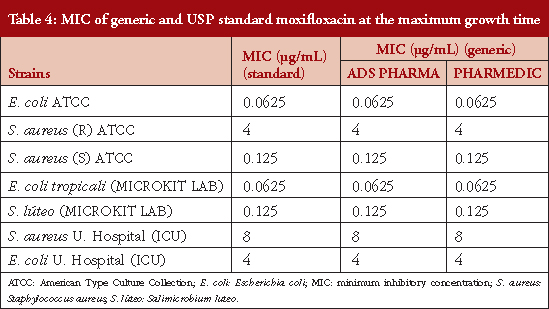
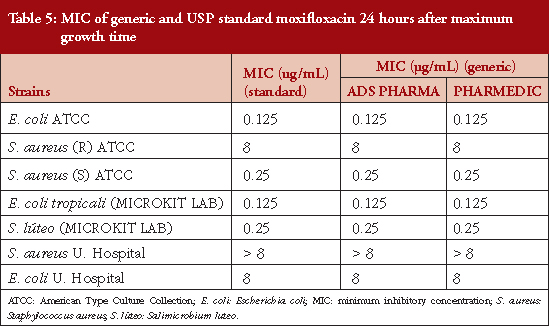
Table 4 shows the MICs at maximum growth time: 0.625, 0.125, 0.0625, 0.125, 8 and 4 μg/mL for E. coli ATCC, S. aureus (R) ATCC, S. aureus (S) ATCC, E. coli tropicali (MICROKIT LAB), S. luteus canariensis (MICROKIT LAB), S. aureus U. Hospital (ICU) and E. coli U. Hospital (ICU) respectively. In Table 5 it is observed that MIC values were doubled for all strains studied at 24 hours after the maximum growth time, indicating that moxifloxacin loses potency by half every 24 hours. This indicates that the MIC for the strains from the intensive care units were found in higher ranges than for those references established in this study.
Discussion
To ascertain whether the concentration of the generic medication and the commercial version are equivalent, the active substance in the two versions was quantified and the results showed that there was no statistical difference between the two medications (p > 0.05). The strength of both the generic and commercial version of the antibiotic medication was measured via microbiological tests which revealed that the quantity of active ingredient in the two medications is similar, i.e. their formulations are similar.
With respect to the growth of the different bacteria strains used in this study, we compared the ATCC strains with those from the MICROKIT SL Laboratories, and the isolates from hospital settings. Here, we observed that, compared to the other strains we used, the latter have greater cellular concentration at the time of maximum growth. This was unlike the MICROKIT Laboratories samples which, although demonstrating the longest time of maximum growth among all strains investigated, showed lower bacterial concentration. The MIC determined in this study, that compared standard moxifloxacin with two generic batches of moxifloxacin against E. coli ATCC and E. coli tropicali, had similar ranges to those observed in the Lai et al. 2007 study which showed an MIC of 4 μg/mL [16]. However, Van Bambeke et al., 2005, reported a range of between 3.5 μg/mL and 8 μg/mL [17].
In the present study, it was found that the MIC for the USP standard moxifloxacin versus the two commercial generic batches of moxifloxacin, when used in combination with sensitive ATCC S. aureus strains, was 0.125 μg/mL, which was greater than that reported by Lai et al. 2007 of 0.032 μg/mL. However, this falls within the range reported by Soussy et al. of 0.16−8 μg/mL and also that in the study by Van Bambeke et al., 2005, who reported a range of 6−9 μg/mL [16, 18]. The results for the MIC of USP standard moxifloxacin versus two commercial generic batches of moxifloxacin active against E. coli tropicali, was less effective than that reported in other studies [19]. This constitutes the first report of antibacterial activity of in vitro generic moxifloxacin for this species in Colombia. Likewise, the result for S. luteus canariensis showed MIC values of 0.125 μg/mL and this is the first report on generic moxifloxacin for this new Colombian species reported. In the supplementary material you can see the certificate of quality control and identification of these new native strains in Colombia from MICROKIT Laboratories. The MIC values reported for strains from the specialized care centre did not exceed those reported by Soussy et al., 2003 of 0.16−8 μg/mL or the study by Van Bambeke et al., 2005, who reported a range of 6−9 μg/mL [17, 18].
The extracted data from the assay of biological activity that was carried out using all the bacterial strains mentioned in this study, shows increased absorbance at 24 hours after maximum growth. This is no greater than that shown in the other studies cited above which could be an alarming signal. Microorganisms were shown to have greater adaptability in the presence of moxifloxacin during this time. When the maximum absorbance taken 24 hours after maximum growth is compared to the period of maximum growth, see Table 5, the MIC is cut in half.
In summary, the therapeutic alternatives of moxifloxacin evaluated in this study produce similar MIC values to those seen in results reported by Sanchez-Hoyos et al., 2018 [20] where, using the same methodology, it was shown that both generic and innovative forms of levofloxacin had comparable values for MIC against the strains isolated from different hospitals and the ATCC reference [20]. However, in their study, strains that were taken from ICUs presented higher MIC values, especially after 24 hours of growth. This indicated an increase in resistance to moxifloxacin by the clinical strains isolated which is associated with the use of both generic and brand-name quinolone medications, as has been reported in recent years.
Conclusions
This study has revealed that the concentrations of the main active ingredient in different commercial generic forms of moxifloxacin formulations marketed in Colombia are in the range established by USP 38 required for the submission of injectable moxifloxacin 400 mg/250 mL. The results for the MIC of both generics were 0.125 μg/mL, 4 μg/mL, and 8 μg/mL for resistant S. aureus, ATCC and E. coli tropicali bacteria, respectively. In this study, all commercial generic and USP standard reference showed similar MIC values to all bacteria evaluated.
Acknowledgements
The authors are indebted to Cartagena University. We also appreciate the support of pharmacists Nohra Navarro and Carlos Sanchez from Cartagena University
Competing interests: The authors declare no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
1Farmabac Research Group, School of Pharmaceutical Sciences, University of Cartagena, Cartagena, Colombia
2Chemistry of Medicinal Research Group, School of Pharmaceutical Sciences, University of Cartagena, Cartagena, Colombia
3Farmabac Research Group, School of Medicine, University of Cartagena, Cartagena, Colombia
Editor’s comment
The aims of the study are admirable, i.e. to confirm the quality of generic moxifloxacin compared to innovator moxifloxacin used in Colombia. But the study is very limited as it includes only one batch of each of two generic drug products; assessment of several batches is necessary if any meaningful conclusions are to be drawn.
A major drawback of the manuscript is that originator moxifloxacin is not included; all comparison is with the USP standard for moxifloxacin which is not a clinical product (it is obtained from a reagent company rather than a pharmaceutical organization).
The findings are exactly as would be expected, but its limitations really restrict its value.
References
1. Miller JN, Miller JC. Estadística y quimiometría para química analítica. Hall P, editor. Pearson Educación; 2002. p. 97-8.
2. Nicholas JM. Clinical development, immunogenicity, and interchangeability of follow-on complex drugs. Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(2):71-8. doi:10.5639/gabij.2014.0302.020
3. Arias Palacios J, Bustamante Ojeda S, Ortiz Gonzalez V, Moya Moreno M. Comparación de la actividad antimicrobiana de meropenem genérico y meropenem innovador por la técnica de micro dilución en cepas resistentes. Rev Cubana Farm. 2015;49(4).
4. Gauzit R, Lakdhari M. Generic antibiotic drugs: is effectiveness guaranteed? Med Mal Infect. 2012;42(4):141-8.
5. Sun HY, Liao HW, Sheng MH, Tai HM, Kuo CH, Sheng WH. Bioequivalence and in vitro antimicrobial activity between generic and brand-name levofloxacin. Diagn Microbiol Infect Dis. 2016;85(3):347-51.
6. Vesga O, Agudelo M, Salazar BE, Rodriguez CA, Zuluaga AF. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob Agents Chemother. 2010;54(8):3271-9.
7. Silva E, Díaz JA, Arias MJ, Hernández AP, de la Torre A. Comparative in vitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administration. BMC Clin Pharmacol. 2010;10(1):3.
8. Bustamante Ojeda SI. Protocolo para la evaluación/comparación de la actividad antimicrobiana antibióticos genéricos y antibióticos innovadores, frente a patógenos clínicos. Facultad de Ciencias; 2015.
9. Finch R. Generic antibiotics, antibiotic resistance, and drug licensing. Lancet Infect Dis. 2010;10(11):754.
10. Hooper DC, Jacoby GA. Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;1354(1):12-31.
11. Pharmacopeia U. United States Pharmacopeia and National Formulary (USP 38–NF 33). Vol Section. 2016;2:35-117.
12. Zaraza Moncayo ZM. Actividad antibacteriana del aceite esencial de la conobea scopariodes frente a cinco cepas bacterianas de interés clínico en Colombia. Repository Universidad del Rosario. 2012;1.
13. Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute Standards Development Policies and Process. M07-A102014.
14. Cattaneo CA, Biasoni EM, Larcher LI, Ruggeri AI, Gómez Khairallah AO. Aplicación de dimensión fractal al estudio de sistemas naturales. Mecánica Computacional. 2009;XXVIII:2021-38.
15. Perilla MJ, Bopp C, Elliott J, Facklam R, Popovic T, Wells J, et al. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world: Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae, Neisseria gonorrhoea, Salmonella serotype Typhi, Shigella, and Vibrio cholerae. 2003.
16. Lai WW, Chu KO, Chan KP, Choy KW, Wang CC, Tsang CW, et al. Differential aqueous and vitreous concentrations of moxifloxacin and ofloxacin after topical administration one hour before vitrectomy. Am J Ophthalmol. 2007;144(2):315-8.
17. Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect. 2005;11(4):256-80.
18. Soussy CJ, Nguyen J, Goldstein F, Dabernat H, Andremont A, Leclercq R, et al. In vitro antibacterial activity of moxifloxacin against hospital isolates: a multicentre study. Clin Microbiol Infect. 2003;9(10):997-1005.
19. Chidiac C, SPILF working group. Update on a proper use of systemic fluoroquinolones in adult patients (ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin, ofloxacin, pefloxacin. SPILF.). Med Mal Infect. 2015;45(9):348-73.
20. Sanchez-Hoyos C, Alviz-Amador A, Duran-Lengua M. In vitro antimicrobial activity of generic and brand-name Levofloxacin against clinical and ATCC strains E. coli and S. aureus. Afr J Pharm Pharmacol. 2018;12(4):52-60.
|
Author for correspondence: Auxiliary Professor Antistio Alviz-Amador, MSc, Farmabac Research Group, School of Pharmaceutical Sciences, University of Cartagena, Cra. 6, No. 36–100 Calle de la Universidad, Cartagena, Colombia |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2018 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.