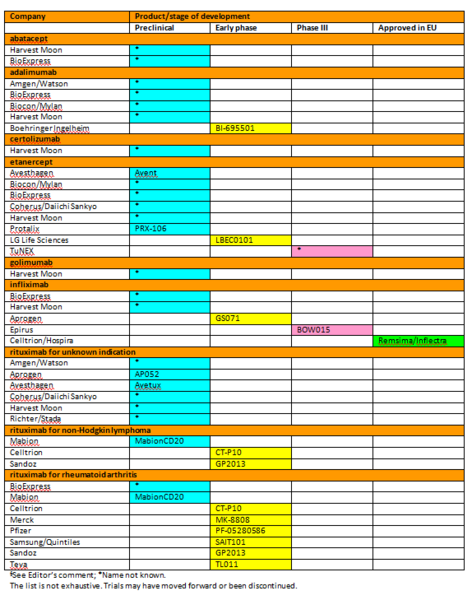
Biosimilars development is a hive of activity, with development of biosimilars for the treatment of rheumatoid arthritis being one of the busiest areas in the biosimilars field, see Table 1 [1].
Table 1: Biosimilars and non-originator biologicals§ in development
±Data collected until 7 November 2014
With so many biosimilars under development, the future could see the prospect of biosimilars being introduced shortly after patent expiry. The patent on Remicade (infliximab) only expires in Europe in February 2015 [2]. Despite this fact, Celltrion/Hospira’s infliximab biosimilar Remsima/Inflectra has already been approved in the European Union in September 2013 [3].
Editor’s Comment
It should be noted that biosimilars approved in countries outside the European Union (EU) might not have been authorized following as strict a regulatory process as is required for biosimilars in the EU. The EMA (European Medicines Agency) regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product.
If you would like to receive a high-resolution copy of Table 1*, please send us an email.
*For profit organizations subjected to a fee
Related articles
Global and domestic biosimilars players increasing
Biosimilars in emerging markets
References
1. Sheppard A. Biological/biotechnological and biosimilars market: the global outlook with special focus on Europe. 12th EGA International Symposium on Biosimilar Medicines; 3–4 April 2014; London, UK.
2. GaBI Online – Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Nov 7]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
3. GaBI Online – Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Nov 7]. Available from:www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
Source: www.gabionline.net