

Submitted: 14 May 2021; Revised: 14 May 2021; Accepted: 3 June 2021; Published online first: 10 June 2021
Medicines regulatory authorities aim to address rising healthcare costs and promote access to medicines worldwide through review and approval of quality generic drug products that are interchangeable with the corresponding reference medicinal product.
The Bioequivalence Working Group for Generics (BEWGG) of the International Pharmaceutical Regulators Programme (IPRP) aims to promote greater collaboration, regulatory convergence and potential mutual reliance on respective bioequivalence assessments in the longer term. At the time of this survey, this group was composed of the regulators of the following jurisdictions: Argentina, Australia, Brazil, Canada, Colombia, the European Union (EU), Japan, Mexico, New Zealand, the Republic of Korea, Singapore, South Africa, Switzerland, Taiwan, the US, as well as the World Health Organization (WHO) which participates as an observer.
In addition to the waivers of in vivo bioequivalence studies (biowaivers) for immediate release (IR) solid oral dosage forms based on the Biopharmaceutics Classification System, i.e. BCS biowaivers, and the biowaivers of additional strengths with respect to the strength for which in vivo bioequivalence has been shown, biowaivers may also apply to certain dosage forms irrespective of the BCS biowaiver criteria.
The BEWGG of IPRP published the current regulatory requirements to waive the in vivo> demonstration of bioequivalence for oral and injectable dosage forms among the regulators of the IPRP BEWGG. The sharing of this information is a first step towards regulatory convergence in this area.
Oral solutions
In vivo bioequivalence studies for oral solutions can be waived in all jurisdictions except Japan, where biowaivers for aqueous solutions are not accepted.
In the case of oily oral solutions, Taiwan and the US would accept a biowaiver, whereas Brazil and the Republic of Korea would not. For other jurisdictions, this would depend on the physicochemical properties of the dosage form. For example, some members of the IPRP BEWGG would require that the type of oil used in the vehicle be the same as that in the reference product.
For other types of oral solutions, a biowaiver would be considered acceptable based on the qualitative and quantitative differences in the non-medicinal ingredients/excipients. Qualitative differences in excipients are acceptable in principle if the excipients are not considered to be critical, i.e. known not to affect the bioavailability of the active ingredient(s), e.g. preservatives, viscosity agents, pH buffers, colorants, flavours, some sweeteners. However, qualitative similarity and remarkably close quantitative similarity would be expected for excipients that enhance absorption, e.g. polysorbate 80. Some members assess the similarity of excipients in oral solutions according to the requirements for BCS-based biowaivers, where non-critical excipients can be modified for oral solutions containing BCS class I drugs, but not for oral solutions containing BCS class II, III and IV drugs where the excipients must be qualitatively the same and quantitatively similar. In Canada, any difference beyond the described criteria should be scientifically justified. Similarly, in the US, the different amount of any excipient should be within US Food and Drug Administration (FDA) inactive ingredients database limits and the new amounts should not be associated with safety or efficacy concerns.
For excipients that are considered critical because they are known to potentially affect the bioavailability of active ingredients by altering the gastrointestinal transit, permeability or stability of the active ingredients, most members do not allow qualitatively changes, but permit minor quantitative changes. In the US and Argentina, critical excipients can be changed qualitatively and quantitatively within certain justified limits. The list of critical excipients is not exhaustive but includes surfactants, e.g. SLS, castor oil ethoxylate, polysorbate 80; sweeteners, e.g. sorbitol and mannitol, excipients that affect transporters, e.g. PEG-400; co-solvents and complexing agents, e.g. cyclodextrins. Each jurisdiction may have different criteria on the types of excipients that are considered critical, and the quantitative differences allowed.
In the case of powders for reconstitution of oral solutions, the same requirements apply because the product is an oral solution at the time of administration.
Oral suspensions
South Africa, Australia and Singapore will consider biowaivers for oral suspensions for systemic action if they have similar (South Africa) or identical (Australia and Singapore) quantitative formulations and the physicochemical equivalence of justified parameters, e.g. polymorphic form, particle size distribution, viscosity, pH and dissolution profiles across the pH range 1.2 to 6.8. In all other jurisdictions, in vivo bioequivalence studies are required.
In the case of locally acting suspensions, biowaivers can be accepted in Brazil and Singapore, and considered on a case-by-case basis in most of the other members if the drug substance is not systemically absorbed. In the US, specific examples where a biowaiver is accepted are sevelamer, colesevelam and cholestyramine. Japan and Mexico do not accept biowaivers for locally acting suspensions.
In the case of powders for reconstitution of oral suspensions, the same requirements apply because the product is an oral suspension at the time of administration.
Soft gelatin capsules
With the exception of Brazil, Canada, Japan and the Republic of Korea, a biowaiver from conducting in vivo bioequivalence studies could be acceptable in the remaining jurisdictions, if the drug substance is in solution inside the capsule and the fill liquid is qualitatively the same and quantitatively similar or identical (depending on the jurisdiction) to that of the comparator product. In the US, for example, a biowaiver could be accepted for products containing omega-3-acid ethyl esters; however, in most cases in vivo studies are required, e.g. products containing progesterone.
Intravenous injections
In vivo bioequivalence studies for simple intravenous solutions for injection or infusion may be waived in all jurisdictions. For Canada, the formulations of the generic and the reference medicinal product should be qualitatively the same and quantitatively essentially the same (excipient variation between products is within ±10% unless data are available to support a wider variation). Any differences beyond the criteria should be scientifically justified. Only preservatives, buffers, antioxidants can be different in the Republic of Korea and the US, while isotonic agents can also be changed in the other jurisdictions. Excipients that may affect disposition and/or safety, e.g. surfactants like Cremophor, should not differ in most of these countries. For Brazil, any excipient can be changed as long as the new excipients are well established for intravenous administration and used in suitable concentrations, but any differences in preservatives, buffers and thickening agents need to be justified.
In Brazil and Canada, a physicochemical comparison is always required. In some jurisdictions, a physicochemical comparison is required in cases of differences in excipient composition, whereas in other jurisdictions, compliance with pharmacopoeial requirements for intravenous solutions is considered sufficient without any comparison with the comparator product.
In the case of powders for reconstitution of intravenous solutions, the same requirements apply because the product is an intravenous solution at the time of administration.
Intramuscular and subcutaneous solutions for injections
All members except Japan accept biowaivers for subcutaneous and intramuscular solutions. In Japan, these waivers are not described in current guidelines, and they are assessed on a case-by-case basis. The US would require in vivo pharmacodynamic studies for certain products to demonstrate similar activities, e.g. dalteparin, enoxaparin, however, other jurisdictions would classify low molecular-weight heparins as biosimilars in this context.
In most members, a biowaiver is possible for oily solutions only if the same oily vehicle is used.
Qualitative and quantitative differences in buffer agents, antioxidants and preservatives are acceptable in principle for all jurisdictions if the differences are scientifically justified. Most members also accept differences in isotonic agents. Excipients such as those affecting viscosity, surfactants and complexing agents should not be changed in most countries. In contrast, Brazil and Argentina assess changes on a case-by-case basis. The requirements for physicochemical comparisons are the same as for intravenous injections described above.
In the case of powders for reconstitution of subcutaneous or intramuscular solutions, the same requirements apply because the product is a solution at the time of administration. In the US and Japan, this is not described in the guidelines. Nevertheless, the US would apply the same principles, while Japan assesses this on a case-by-case basis.
Intramuscular and subcutaneous suspensions for injections
For intramuscular and subcutaneous suspensions for injection, a biowaiver is not acceptable in principle in any of the jurisdictions. However, in rare instances, a biowaiver may be acceptable, e.g. azacytidine, as specified in the product-specific guidances from FDA and Brazil. Azacitidine powder for suspension for injection products have been waived in Australia, Canada, the EU and Switzerland as exceptional cases, since azacitidine is not completely soluble at room temperature (25°C), but rather is soluble at 37°C, when all in vitro tests have shown to be similar.
Emulsions for intravenous injection
In most participating countries, a biowaiver is possible for emulsions for intravenous injection, e.g. aprepitant, clevidipine and propofol, whereas a biowaiver is not possible in Brazil, Japan and Taiwan. In Australia, Canada, New Zealand, Singapore and South Africa the excipient composition should be qualitatively the same and quantitatively very similar to that of the comparator product, while minor differences, e.g. antioxidants, have been accepted in the EU, the Republic of Korea and Switzerland. The biowaiver is based on physicochemical comparability of droplet size distribution in the dispersed lipid phase, viscosity/rheological properties, pH, osmolarity, specific gravity, surface properties such as zeta potential.
Biowaiver requirements for such products are not currently described in guidance documents from Argentina, Canada, Colombia and WHO.
Micellar solutions for intravenous injection
The US does not consider injectable micelles as a distinct dosage form; instead, they are designated as injections or injectable solutions. In most countries, a biowaiver is possible for micellar solutions for injection, e.g. docetaxel micellar solutions, whereas in Argentina and Brazil, the biowaiver requirements are not addressed in current guidelines and applications are assessed case-by-case. In those jurisdictions where a biowaiver is acceptable, the excipient composition should be qualitatively the same and quantitatively very similar, although minor qualitative differences in buffer agents, antioxidant and preservatives are accepted. In addition, some jurisdictions could accept qualitative changes in the co-solvents if they are not considered critical, e.g. alcohol and PEG. The biowaiver is based on physicochemical comparability of critical micellar concentration (CMC), micelle size distribution, solubilisation capacity (free and bound amounts) and pH, osmolarity and viscosity. In Japan and Taiwan, biowaivers are not acceptable for such products.
A waiver from in vivo demonstration of bioequivalence may be applied to several orally administered and systemically acting dosage forms like oral solutions, oral suspensions and soft gelatin capsules, and some systemically acting parenteral dosage forms like intravenous injections, subcutaneous and intramuscular injections, emulsions for injection and micellar solutions for injection. The survey showed that the biowaiver criteria for these dosage forms are diverse among the participating BEWGG member organizations and that the complexity of the dosage forms correlates with the risks associated with accepting biowaivers. This may explain why the biowaiver requirements for the more complex dosage forms, e.g. suspensions, micellar injection, tend to be more variable among the participating members, while there is more similarity in requirements for the less complex dosage forms, e.g. oral solutions, IV injections, as there are less risk factors to consider that may influence the safety and efficacy of the product. The sharing of this information is a first step towards regulatory convergence in this area since, for some dosage forms, large differences between members of the BEWGG of the IPRP have been identified. The next steps should involve identifying areas that could be harmonized based on sound scientific justifications. Convergence in this area would be useful for pharmaceutical companies developing generic medicinal products for more than one of these jurisdictions.
Article [1] abstracted by Alfredo García-Arieta, World Health Organisation (WHO) Prequalification of Medicines Programme and Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), División de Farmacología y Evaluación Clínica, Subdirección General de Medicamentos de Uso Humano, C/ Campezo 1, Edificio 8, ES-28022 Madrid, Spain; and Clare Rodrigues, Health Sciences Authority (HSA), Health Products Regulation Group, Medicinal Products Pre-Marketing Cluster, Therapeutic Products Branch, Singapore.
Competing interests: None
Provenance and peer review: Commissioned; internally peer reviewed.
References
1. Garcia Arieta A, Simon C, Tam A, Mendes Lima Santos G, Freitas Fernandes EA, Rodríguez Martínez Z, et al. A survey of the regulatory requirements for the waiver of in vivo bioequivalence studies of generic products in certain dosage forms by participating regulators and organisations of the International Pharmaceutical Regulators Programme. J Pharm Pharm Sci. 2021;24:113-26.
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2021 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/an-iprp-survey-of-the-regulatory-requirements-for-the-waiver-of-in-vivo-bioequivalence-studies-of-generic-medicinal-products-in-certain-dosage-forms.html
Submitted: 4 March 2019; Revised: 25 April 2019; Accepted: 27 April 2019; Published online first: 30 April 2019
The availability of quality generic drug products plays an increasingly important role in promoting access to medicines worldwide and in helping to address rising healthcare costs. This, however, has led to significant pressures on medicines regulatory authorities charged with the review and approval of these products.
The Bioequivalence Working Group for Generics (BEWGG) of the International Pharmaceutical Regulators Programme (IPRP, previously International Generic Drug Regulators Programme) aims to promote greater collaboration, regulatory convergence and potential mutual reliance on respective bioequivalence assessments in the longer term. This group is composed of the following regulators/agencies: Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, ANVISA, Brazil), Federal Commission for the Protection against Sanitary Risks (Comisión Federal para la Protección contra Riesgos Sanitarios, COFEPRIS, Mexico), European Commission/European Medicines Agency (EC/EMA), Health Canada (HC), Health Sciences Authority (HSA) Singapore, National Food and Drug Surveillance Institute (Instituto Nacional de Vigilancia de Medicamentos y Alimentos, INVIMA, Colombia), South African Health Products Regulatory Authority (SAHPRA), Medsafe (New Zealand), Ministry of Food and Drug Safety (MFDS, South Korea), Pharmaceuticals and Medical Devices Agency (PMDA, Japan), Swissmedic (Switzerland), Taiwan Food and Drug Administration (TFDA), Therapeutic Goods Administration (TGA, Australia), United States Food and Drug Administration (US FDA), as well as an observer from the World Health Organization (WHO).
The acceptance of foreign comparator or reference products is one of the topics addressed in the BEWGG since it could be considered the most limiting factor for the development and regulatory assessment of generic medicines marketed globally. Generics companies commonly have to repeat bioequivalence studies with the respective local comparator products of each country or jurisdiction because regulatory agencies are either unaware if the comparator product from other countries is the same product as their own or, if this is known, are unable to use that information for reasons of confidentiality and/or legal restrictions. This results in the duplication of efforts by regulators and industry alike, as well as unnecessary risks to study subjects.
The BEWGG of IPRP published the current regulatory requirements with respect to the acceptability of foreign comparator products of oral dosage forms among the regulators/agencies that participate actively in the BEWGG [1] to identify those participants that may accept a foreign comparator product under certain conditions. The sharing of the relevant information contained in their respective regulatory guidance documents and policies is a first step towards regulatory convergence in this area.
Brazil, Colombia, the European Union Member States, Japan, Mexico, South Korea and the US do not accept foreign comparators and accept bioequivalence studies involving only their local comparator products, i.e. comparator products sourced from within their corresponding jurisdictions. In contrast, Australia, Canada, New Zealand, Singapore, South Africa, Switzerland and Taiwan accept studies with foreign comparator products under certain conditions. Only WHO lists specific comparators from different countries for the Prequalification of Medicines and for developing generics of essential medicines.
Most participants limit the origin of the foreign comparator to countries with a comparable regulatory system. While WHO, South Africa and Switzerland accept comparator products from a list of countries, Australia, Canada and New Zealand have not defined the countries. On the other hand, Singapore and Taiwan do not impose any restrictions relating to the comparability of regulatory systems or agreements with the originating country or jurisdiction. Instead, emphasis is placed on comparing the manufacturing sites of the foreign and local comparator products. Some jurisdictions also have requirements at the company level. Australia, Canada, Singapore, South Africa, Switzerland and Taiwan require that the foreign comparator and the local comparator have to be marketed by the same corporate entity, although Australia, New Zealand, Singapore and South Africa also accept a different corporate entity if there is a licensing arrangement between the local and the foreign companies.
Some countries have specific restrictions specific to drug substance or drug product properties which additionally govern the situations in which foreign comparator products can or cannot be used. For example, in terms of drug substance properties, Australia, Canada and Singapore do not accept foreign comparator products containing narrow therapeutic index drugs (NTIDs) or drugs that require patient monitoring in order to avoid the consequences of under- or over-treatment. Australia and Canada do not accept foreign comparators of drugs with complicated pharmacokinetics, variable or incomplete absorption or absorption window or substantial first pass metabolism. Canada also limits the acceptance of foreign comparator products to those that contain highly soluble drugs. In contrast, there are no exclusion criteria with respect to drug substance properties in New Zealand, South Africa, Switzerland and Taiwan. In terms of drug product properties, Canada only accepts foreign comparator products for immediate release formulations, while Australia accepts immediate and enteric/delayed release products and may accept a foreign comparator product for sustained release products on a case-by-case basis. On the other hand, New Zealand, Singapore, South Africa, Switzerland and Taiwan do not have restrictions based on the release profile of the drug product.
Most participants require details regarding the source of supply, batch information and the foreign innovator company. In addition, the labelling and the Certificate of Analysis (CoA) of the foreign comparator product batch employed in the bioequivalence study is commonly required. Notably, Canada requires that product samples in their original container closure systems be made available upon request. Also, in New Zealand, reduced data requirements are applied to foreign comparator products sourced from the Australian market, provided evidence of harmonisation of the comparator product between the two markets can be demonstrated. Acceptable evidence supporting harmonisation between the New Zealand and Australian innovator products includes copies of the labelling or package inserts that demonstrate co-marketing in both countries.
The formulations of the local and the foreign comparator product should be compared for all participants. All seven countries require a qualitative comparison of the excipient composition. For Australia, Canada and New Zealand the local and the foreign comparator product have to have the same size, weight, shape, colour, scoring and type of coating. In contrast, Taiwan only requires them to have the same size, weight and type of coating, Switzerland requires them to have only the same size and weight, and Singapore and South Africa may accept differences as long as the release type is the same. Australia, New Zealand, Switzerland and Taiwan also require some physicochemical testing such as Fourier transform infrared spectra, near infrared spectra and powder X-ray diffraction spectra. All of these participants except South Africa also require the foreign comparator employed in the bioequivalence study to have the same strength as the local one. Interestingly, Australia is the only jurisdiction that requires a quantitative analysis in sufficient batches to determine batch-to-batch variability (often two to three batches) using validated test methods. In all these seven countries, it is necessary to demonstrate that the local and the foreign comparator product have similar dissolution profiles across the physiological pH range with 12 individual units per product. However, if it can be shown that the manufacturing site of the foreign and local comparator products are the same, then comparative dissolution data are not required by Singapore and Taiwan. Similarly, in New Zealand, if the foreign comparator product is sourced from the Australian market and harmonisation between the comparator products from New Zealand and Australia has been established, then physicochemical testing, e.g. dissolution, FTIR (Fourier-transform infrared spectroscopy) and XRD (powder X-ray diffraction), is not required.
These results demonstrate that the acceptability of foreign comparator products among BEWGG participants is not harmonised. While some countries do not accept this practice, other countries are open to the possibility of accepting foreign comparator products under certain conditions. However, the requirements to demonstrate that the foreign comparator and the local comparator are the same product in these countries differ widely. Importantly, for the WHO Prequalification Programme, both the European and the US comparator products are considered to be valid comparators because they ensure ‘prescribability’, i.e. an adequate safety and efficacy profile, even if they might exhibit different bioavailability and, consequently, the generics approved based on a comparison with the US comparator may not be ‘switchable’ with the generics approved based on a comparison with the European comparator.
It is interesting to note that the criteria of the BEWGG members appear to correlate reasonably well with the market size of the jurisdiction. Those jurisdictions with a large population (more than a hundred million inhabitants) require bioequivalence studies with their local comparator and generics companies are able to conduct the studies because they are profitable with those market sizes. In contrast, those countries with a smaller market size are open to accept bioequivalence studies with foreign comparators if these appear to be the same as the local ones. In those countries with middle size, the purchasing power of the population and the availability of local manufacturers may also play a role – South Africa (53 million) may accept foreign comparators, whereas Colombia (49 million) and South Korea (51 million) do not. Similarly, Australia and Canada, with 25 and 36 million inhabitants, respectively, impose important limitations for the acceptability of the foreign comparator. In countries that are smaller and with lower purchasing power than those participating in the BEWGG, it can be expected that generic drug products would not be developed with the local comparator products because those markets are not considered profitable enough for generics companies, e.g. Zimbabwe. These countries generally align with WHO and accept studies conducted with comparators from founding International Conference on Harmonisation (ICH) countries under the assumption that they will be the same in all the ICH countries, without any additional requirements as long as this foreign comparator is marketed by the innovator company.
Accepting foreign comparator products may bring about public health benefits by increasing the availability of generic medicines and thereby reducing healthcare costs. By decreasing the number of bioequivalence studies that industry is required to perform, regulators are able to lower barriers to generic drug applications while still maintaining the integrity and standards of safe, effective and quality generic drug products available for the people within their jurisdiction. On the other hand, the acceptance of foreign comparators in bioequivalence (BE) studies does have some risk in that the product may not be the same as the local comparator and may thus bring about switchability issues. Where a jurisdiction accepts the use of a foreign comparator, they apply restrictions to control the risk associated with switchability issues. Since greater restrictions on the use of a foreign comparator may also increase barriers to market entry for generic drug products, each jurisdiction must determine the appropriate approach for their healthcare system.
Those countries that require the exclusive use of their local comparator do so to ensure ‘switchability’ of all approved generics in their markets. For countries whose regulatory systems allow them to accept foreign comparator products, the acceptability of foreign comparator products is complicated by the absence of data confirming that the foreign comparator is identical to the local one. The sharing of confidential business information such as the composition, manufacturing process and specifications of the comparator products between regulators is not allowed by the present legislation of most countries. To overcome this obstacle, the legal systems would need to be modified to allow regulatory agencies to share needed confidential information about comparator products with other regulatory agencies.
Another potential approach to enhanced acceptance of foreign comparator products through sharing of information could involve the use of an independent third party, such as WHO, who could determine the similarity of comparator products. In this scenario, it would not be necessary to require the generics company to perform any comparability tests and restrictions on the acceptance of a foreign comparator could likely be eliminated.
Overall, in the presence of barriers which inhibit the acceptance of foreign comparator products, it will continue to be necessary to perform bioequivalence studies using the local comparator, or in some cases, to conduct a battery of in vitro tests to demonstrate the similarity/identity of the local comparator product to the foreign comparator product used in a bioequivalence study.
Competing interests: None.
Provenance and peer review: Article abstracted by Dr Alfredo García Arieta, Clare Rodrigues and Christopher Crane from a published paper [1]; internally peer reviewed.
Reference
1. García Arieta A, Simon C, Lima Santos GM, Calderón Lojero IO, Rodríguez Martínez Z, Rodrigues C, et al. A survey of the regulatory requirements for the acceptance of foreign comparator products by participating regulators and organizations of the International Generic Drug Regulators Programme. J Pharm Pharm Sci. 2019;22(1):28–36.
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2019 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/regulatory-requirements-for-the-acceptance-of-foreign-comparator-products-in-the-participants-of-the-international-generic-drug-regulators-programme.html
Author byline as per print journal: Luther Gwaza1,2, BPharm, MPhil; John Gordon3, PhD; Henrike Potthast4, PhD; Marc Maliepaard5, PhD; Jan Welink5, Hubert Leufkens1,5, PhD; Matthias Stahl6, MD; Alfredo García-Arieta7, PhD
|
Abstract: |
Submitted: 21 March 2016; Revised: 11 April 2016; Accepted: 11 April 2016; Published online first: 25 April 2016
Direct comparison within well-designed and well-conducted randomized controlled trials (RCT) is the gold standard for comparing health interventions. The usual practice to obtain marketing authorization for new health interventions is to compare them to placebo, or standard of care, but not with all available health interventions. Some interventions are developed simultaneously, thus it is not feasible to perform comparison between them during this development phase to support marketing authorizations. Moreover, in some cases, there is a relatively large number of available interventions making direct comparison through RCTs between them impractical. Therefore, in these instances where multiple interventions exist, there is often lack of, or insufficient, evidence of direct comparison (head-to-head comparison) to evaluate relative effectiveness of all the available health interventions; indirect comparisons are then employed [1–3].
Indirect treatment comparison is defined as an evaluation of different health interventions using information from independent studies. This is useful when there are no data on direct comparison, or to provide supplementary evidence when the data from direct comparison are insufficient [4]. Indirect comparison can be categorized into naïve (unadjusted), informal indirect comparison, and adjusted indirect comparison [3]. Naïve indirect comparison evaluates the data from the independent studies as if the data are from the same study ignoring the between-study variance. For this reason, evidence from naïve indirect comparison is equivalent to observational studies, prone to bias and it may over- or underestimate the treatment effect; thus this approach is not recommended for analysing data from RCTs [2–4]. In informal indirect comparison, the results from the independent studies are compared directly, and relative effects or statistical significance are not formally calculated [3]. Adjusted indirect treatment comparison evaluates different treatments tested in independent studies modified based on the results of their direct comparison with a common control, partly preserving the power of RCTs [3] without the added cost of actual direct RCT comparison. However, adjusted indirect comparisons are less precise as reflected in wider confidence intervals [4], thus wherever possible direct comparisons should be performed.
Figure 1 illustrates the direct and indirect comparison of health interventions. Suppose there are four different treatments, A, B, C and D compared in three different trials. If treatment A was compared in an RCT with treatment B, treatment C in another RCT with treatment B, and treatment C with D in another RCT, adjusted indirect treatment comparison can be used to compare treatment A and C since both were tested in two independent trials with the common treatment B. Likewise, treatment B and D can be compared using adjusted indirect comparison since both were compared in direct comparison with common treatment C.
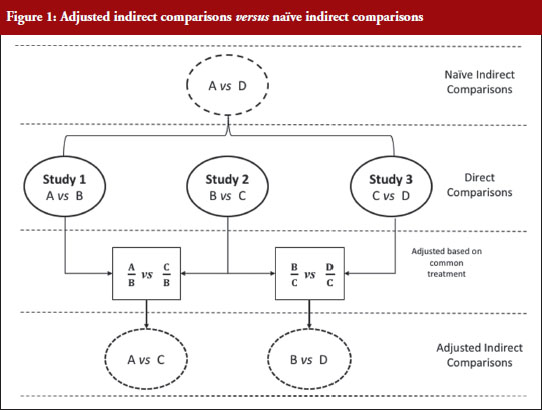
Generic medicines are approved nationally following the expiry of patents or market exclusivity period for the innovator products based on demonstration of bioequivalence with the innovator product. A bioequivalence study comparing a generic and an innovator drug is a form of direct comparison. With time, the number of approved generic medicines per active substance generally increases. Thus, requirement for direct comparison through bioequivalence studies between generics is impracticable since several generics of the same drug are licensed at various times, often without knowledge of the other generics under development. For this reason, adjusted indirect treatment comparison is a useful approach to identify those generic drug products whose interchangeability can be assured, by comparing the different generic drug products that have been demonstrated to be bioequivalent with the same reference product, to support or ensure switchability in clinical practice without concerns on efficacy or safety due to the switching.
Several methods are available for performing indirect comparisons: (1) naïve or unadjusted indirect comparisons; (2) statistical methods using aggregate data such as simple weighted combination of separate estimates as suggested by Bucher et al. [1] (adjusted indirect comparison); (3) modelling approaches based on the individual patient data (meta-regression); and (4) mixed treatment comparisons based on Bayesian statistics (logistic regression) [2, 5].
In meta-regression analysis, the estimated difference between the groups (treatment effect) is modelled as a function of one or more study characteristics as the predictor variable. Estimated treatment effect in each study is weighted according to the inverse of its variance. A simple approach for meta-regression is weighted linear regression, and the residual heterogeneity is estimated using random effects. Meta-analysis can be performed using the fixed-effects to describe the residual heterogeneity. The key assumption for fixed effect meta-analysis is that the different trials estimated the same effect, for example, the effect of treatment A relative that of treatment B. Regression methods such as logistic regression can be used to perform indirect comparison using the generalized linear models and individual patient data. With regression modelling, one can adjust for other variables available for each study. While full individual patient data, which are required for logistic regression for indirect comparison, or the estimated treatment effect, its variance and covariates for each trial, which are necessary to perform meta-regression, are rarely publicly available, adjusted indirect comparison is performed using the summarized data available in published articles and approved product labelling. It is the simplest and most appropriate methodological approach when only two interventions are to be compared indirectly as it is the case for bioequivalence studies [6].
When using summarized data extracted from published data, first the data are extracted or calculated using appropriate summary statistics, e.g. confidence intervals, mean ratios, for each set of studies. For bioequivalence studies, the extracted data are study products, sample sizes preferably in each sequence, confidence intervals and study design (fasting or fed study, parallel or crossover, single or multiple dose studies). The confidence intervals of the bioequivalence studies are converted to log scale and used to estimate the point estimate and standard error of the treatment effects. Lastly, the data are combined to provide an overall comparison. The standard statistical result, i.e. the variance of the difference between the two independent estimates, is sum of the two variances (variance is square of the standard error), which is similar to a 2-sample t-test. Thus, using the illustration in Figure 1, if you have the two estimated effects for A υs B and B υs C as θAB and θBC, respectively, the effect of the comparison A υs C is estimated as θAC = θAB − θBC and var (θAC) = var (θAB) + var (θBC) [1]. The scale of the effect θ relates to the scale on which the data would be analysed, such as risk difference, log risk ratio, log odds ratio for binary data, the means, mean difference, mean change for continuous data and log hazard ratio for time-to-event data. In bioequivalence studies, the pharmacokinetic outcome measure is continuous data, and the effect θ is the ratio of log-transformed geometric means of the treatments, e.g. A and B (point estimate). The 90% confidence interval (CI) of the ratio of the log-transformed geometric means is the standard statistical result. Therefore, the 90% CI for the indirect comparison 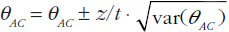 , where z/t in this equation is the z value of standardized normal distribution or the t value of the Student’s t distribution that corresponds to the desired level of confidence (90% in case of bioequivalence studies) and the degrees of freedom in the case of the Student’s t distribution.
, where z/t in this equation is the z value of standardized normal distribution or the t value of the Student’s t distribution that corresponds to the desired level of confidence (90% in case of bioequivalence studies) and the degrees of freedom in the case of the Student’s t distribution.
We explored the different approaches for calculating adjusted indirect comparisons [7]. In this study, we compared six methods that can be used to calculate the width of the confidence intervals based on z distribution (z0.9) or Student’s t distribution (t0.9, d.f.). Four methods that assumed small sample sizes with Student’s t distribution are: (a) Chow and Liu meta-analysis method [8], which assumes all studies had 2 × 2 crossover design and homogeneity of the distribution of reference product data in all studies; (b) homoscedastic method which assumes homogenous variances; (c) heteroscedastic method which assumes heterogeneous variances; and (d) the pragmatic approach which does not require the assumption of homogeneity of variances between studies with small sample sizes. The two methods which assumes large sample sizes with a standardized normal distribution (z0.9) are: (a) Chow and Shao meta-analysis method [9]; and (b) the z-distribution method with no assumption on homogeneity of variances [10].
We concluded that although the differences were minor, the homoscedastic method is recommended, unless there are clear differences in variances, because it is the most conservative approach for estimating the confidence intervals for adjusted indirect comparisons.
We investigated the differences in bioavailability between generics prequalified by the World Health Organization (WHO) using adjusted indirect comparisons [7, 11]. These studies investigated a diverse group of products from the antimalarials artemether/lumefantrine [7], first-line antituberculosis drugs, [11] and first-line antiretroviral drugs with a total of 394 indirect comparisons. In contrast to the ±20% acceptance range used for direct comparisons, a ±30% acceptance range is proposed for adjusted indirect comparisons [7, 11], due to the limited precision of indirect comparisons [1, 4].
First, these studies demonstrated the utility of adjusted indirect treatment comparison to compare the bioavailabilities between generic drug products that had been compared with the same reference product in direct comparisons. Second, the outcome of these comparisons indicate that antimalarial artemether/lumefantrine, first-line anti-tuberculosis, and first-line antiretroviral generics prequalified by WHO can be interchanged without any safety and efficacy concerns in clinical settings. Although some comparisons were outside the conventional acceptance limits of ±20% for direct comparisons, there were no generic–generic comparisons outside the ±30% for indirect comparisons, except one comparison for efavirenz Cmax. Failure to show equivalence within a ±30% acceptance range in one out of 394 adjusted indirect comparisons should be interpreted as insignificant number since it is less than 0.3% of the comparisons.
The results obtained with the prequalified generics are consistent with the outcomes reported elsewhere using data from other regulatory authorities [12–14]. Herranz et al. showed that exposures obtained with generic tacrolimus formulations in the Spanish market were within the ±20% acceptance range based on adjusted indirect treatment comparisons [12]. In addition, results from adjusted indirect comparisons were consistent with those from direct comparisons for multiple generic formulations of gabapentin products marketed in The Netherlands [13]. Using data from bioequivalence studies submitted to the Dutch Medicines Evaluation Board (CBG-MEB) for atorvastatin, bicalutamide, naratriptan, olanzapine, perindopril, venlafaxine, cyclosporine, tacrolimus and mycophenolate mofetil, Yu and colleagues showed that in 80% of the cases the indirect comparisons between generics fulfilled the conventional acceptance limit of ±20%, while the remainder were within ±30% [14]. A point estimate constraint in the bioequivalence studies may be relevant for drugs with a narrow therapeutic index, e.g. cyclosporine and tacrolimus, where switching between generics of these drugs is not restricted. Generally, narrow therapeutic drugs are usually assessed with a narrowed acceptance range, e.g. 90.00–111.11%, to ensure interchangeability with the reference [15–17].
We observed that assurance regarding interchangeability of two generic drug products is reduced when either the point estimate ratios in the original studies are shifted from unity by more than 5% or when the width of the 90% confidence interval is large in the direct comparisons [11]. Therefore, we investigated the influence of point estimate, variability of the pharmacokinetic parameters (Cmax and AUC), and the sample size in the original studies on the ability to demonstrate bioequivalence between generics in the adjusted indirect comparisons [18]. However, sample size and variability are not independent since the sample size is calculated based on the expected variability and the desired statistical power. Thus, statistical power is the most relevant parameter for consideration in the computations.
We calculated the outcome of adjusted indirect comparisons for 14,592 scenarios using 57 possible differences between point estimates from 0% to 14% and 16 possible study powers from 50% to 99.99%. The study results illustrated that demonstrating bioequivalence within the conventional acceptance limits of 80–125% by means of adjusted indirect comparisons is only possible if the difference between the point estimate is small (< 5%) for any sufficiently powered study (> 80%). Furthermore, even when both studies are overpowered, the difference cannot be larger than 14%. This study showed that in cases where generic–generic switching maybe of concern, regulators might consider a point estimate constraint in the original studies.
The variance for the adjusted indirect comparison is additive, 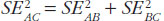 , thus, the major limitation of adjusted indirect comparisons of bioequivalence studies is the reduced precision. On one hand, the inability to show bioequivalence by means of indirect comparisons is not proof of inequivalence, but it may be simply that there is not enough statistical power to make this conclusion. On the other hand, when bioequivalence is shown within the conventional acceptance limits for indirect comparisons despite the reduced statistical power, we can consider not only that the generic drug products are bioequivalent but also very similar.
, thus, the major limitation of adjusted indirect comparisons of bioequivalence studies is the reduced precision. On one hand, the inability to show bioequivalence by means of indirect comparisons is not proof of inequivalence, but it may be simply that there is not enough statistical power to make this conclusion. On the other hand, when bioequivalence is shown within the conventional acceptance limits for indirect comparisons despite the reduced statistical power, we can consider not only that the generic drug products are bioequivalent but also very similar.
The validity of indirect comparisons is dependent on the methodological quality and assumptions. Similarity of trials involved in adjusted indirect comparisons should be carefully assessed to ensure that there are no important differences between the trials under comparison in aspects that could bias the estimated formulation effect. Although standard requirements are applied on the design, conduct and analysis of results of the bioequivalence studies submitted for the prequalification of generics [15], in some cases different study designs might be employed. For example, metabolite versus parent as the analyte, or plasma versus urine as the biological fluid collected for analysis, or multiple versus single dose studies. Studies with these different study designs cannot be compared because the formulation effect cannot be expected to be the same between them. However, we consider results from conventional 2 × 2 crossover designs and replicate designs as combinable. There is no consensus on whether parallel and crossover trials should be combined in indirect comparisons [2], however, this may be possible if the participants and interventions are comparable. In all the analyses performed, all the studies were crossover trials.
In contrast to adjusted indirect comparisons of efficacy trials, confidence in the methodological quality and similarity of adjusted indirect comparisons of bioequivalence studies is assured because of the general consistency in the basic design of these studies. For instance, the characteristics of participants in bioequivalence studies are commonly defined, i.e. usually healthy adult male and/or female volunteers within 18–55 years of age, which controls for the differences in baseline characteristics between treatment groups, whereas differences in disease state in efficacy trials is of concern. The objective of bioequivalence studies is to evaluate formulation differences and external validity of the results is based on the assumption that the effect of the drug in the target populations, i.e. patients would be the same for the test and reference. Nevertheless, in some cases, subject–by–formulation interaction could occur, e.g. when one formulation has excipients that are not tolerated by specific subgroups of patients that are not present in the reference formulation. This is often mitigated by the regulatory requirement to declare such excipients on the product label. Non-randomization of the trials can confound the results if other differences between the treatment groups are linked to the outcomes. Randomization ensures that like is compared with like, i.e. that there are no differences between the groups in any factors other than the intervention itself, in this case, formulation effects. In the bioequivalence studies considered in the analysis of the prequalified generics, subjects were randomized in the allocation of sequence.
Despite the utility of the indirect comparisons, the evidence from such analyses should be interpreted with caution. The internal validity of direct comparisons should be carefully evaluated to reduce bias. In the analysis of the prequalified generics, methodological quality of the studies was not assessed as part of the adjusted indirect comparisons since only the generics that were prequalified were included in the analysis. The prequalification process entails stringent assessment including inspection of the contract research organizations at which the studies were performed, thus providing assurance of the quality of the prequalified product. Therapeutic doses are usually standardized as highest available strength, although in some cases the studies used lower doses. Nonetheless, the results are reported as mean ratios, thus the effect of dose on the outcomes is negligible. The studies were all single dose studies with the same outcome measure of pharmacokinetic parameters Cmax and AUC in all the studies, estimated using validated software. The parent compound in plasma was analysed using validated bioanalytical methods. Despite the general consistency with the bioequivalence studies used in the adjusted indirect comparisons, changes in the requirements over time encompassing several revisions of the guidelines could potentially impact on the methodological quality between studies conducted at different time points; this may be corrected by the use of the same reference product in the different bioequivalence studies. Only the studies using a common reference product as listed by the WHO Prequalification of Medicines Programme were compared in the adjusted indirect comparisons. Though US and European reference products are both accepted in the WHO Prequalification of Medicines Programme, and no distinction was made in the analysis, in some cases these are not the same due to different manufacturing sites and different excipients. However, it is assumed that these products are bioequivalent to the pivotal clinical batch used for gaining marketing authorizations in both jurisdictions.
In conclusion, adjusted indirect comparison is a useful tool to compare relative bioavailabilities between generics that have been compared with a common reference in direct comparison to ensure interchangeability between the generics. The investigated antimalarial – artemether/lumefantrine, first-line antituberculosis and antiretroviral generic drug products prequalified by WHO were considered interchangeable without safety and efficacy concerns. We have also demonstrated that the ability to show bioequivalence between generic drug products by means of indirect comparisons depends on the difference between the point estimates of the bioequivalence studies, which is the point estimate of the indirect comparison, and the power of the bioequivalence studies that are combined. In this respect, concluding equivalence in the indirect comparison within the conventional acceptance limits of 80–125% is only possible when: (a) point estimate difference between generics are low (< 5%) for any sufficiently powered study (> 80%); or (b) the differences do not exceed 14% when both studies are overpowered. Therefore, in cases where it is important to ensure generics interchangeability, the regulatory authorities may consider a point estimate constraint in the original bioequivalence studies. In the general case, due to the reduced precision of indirect comparison, a slightly wider acceptance limits (± 30%) is proposed for indirect comparisons.
Competing interests: The authors declare no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Luther Gwaza1,2, BPharm, MPhil; John Gordon3, PhD; Henrike Potthast4, PhD; Marc Maliepaard5, PhD; Jan Welink5, Hubert Leufkens1,5, PhD; Matthias Stahl6, MD; Alfredo García-Arieta7, PhD
1Utrecht Institute for Pharmaceutical Sciences (UIPS), Utrecht, The Netherlands
2Medicines Control Authority of Zimbabwe, 106 Baines Avenue, Harare, Zimbabwe
3Division of Biopharmaceutics Evaluation 2, Bureau of Pharmaceutical Sciences, Health Canada, Locator 0201C, 101 Tunney’s Pasture Driveway, Ottawa, ON K1A 0K9, Canada
4Federal Institute of Drugs and Medical Devices, 3 Kurt-Georg-Kiesinger-Allee, DE-53175 Bonn, Germany
5Medicines Evaluation Board, 500 Graadt van Roggenweg, NL-3531 AH Utrecht, The Netherlands
6Group Leader Medicines Assessment Prequalification Team – Medicines, HIS/EMP/RHT, World Health Organization, 20 Avenue Appia, CH-1211 Geneva 27, Switzerland
7División de Farmacología y Evaluación Clínica, Departamento de Medicamentos de Uso Humano, Agencia Española de Medicamentos y Productos Sanitarios, 1 Calle Campezo, Edificio 8, Planta 2 Oeste, ES-28022 Madrid, Spain
References
1. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683-91.
2. Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, Bradburn M, Eastwood AJ; International Stroke Trial Collaborative Group. Indirect comparisons of competing interventions. Health Technol Assess. 2005 Jul;9(26):1-134, iii-iv.
3. Song F. What is indirect comparison? What is …? series. February 2009.
4. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ.2003; 326(7387):472.
5. Sutton AJ, Higgins JP. Recent developments in meta-analysis. Stat Med. 2008;27(5):625-50.
6. Schöttker B, Lühmann D, Boulkhemair D, Raspe H. Indirect comparisons of therapeutic interventions. GMS Health Technol Assess. 2009;5.
7. Gwaza L, Gordon J, Welink J, Potthast H, Hansson H, Stahl MM, García-Arieta A. Statistical approaches to indirectly compare bioequivalence between generics: a comparison of methodologies employing artemether/lumefantrine 20/120 mg tablets as prequalified by WHO. Eur J Clin Pharmacol. 2012;68(12):1611-8.
8. Chow SC, Liu J. Meta-analysis for bioequivalence review. J. Biopharm Stat. 1997;7(1):97-111.
9. Chow SC, Shao J. Bioequivalence review for drug interchangeability. J Biopharm Stat. 1999;9(3):485-97.
10. Krauss GL, Caffo B, Chang YT, Hendrix CW, Chuang K. Assessing bioequivalence of generic antiepilepsy drugs. Ann Neurol. 2011;70(2):221-8.
11. Gwaza L, Gordon J, Welink J, Potthast H, Leufkens H, Stahl M, et al. Adjusted indirect treatment comparison of the bioavailability of WHO-prequalified first-line generic antituberculosis medicines. Clin Pharmacol Ther. 2014;96(5):580-8.
12. Herranz M, Morales-Alcelay S, Corredera-Hernández MT, de la Torre-Alvarado JM, Blázquez-Pérez A, Suárez-Gea ML, et al. Bioequivalence between generic tacrolimus products marketed in Spain by adjusted indirect comparison. Eur J Clin Pharmacol. 2013;69(5):1157-62.
13. Yu Y, Teerenstra S, Vanmolkot F, Neef C, Burger D, Maliepaard M. Interchangeability of gabapentin generic formulations in the Netherlands: a comparative bioavailability study. Clin Pharmacol Ther. 2013;94(4):519-24.
14. Yu Y, Teerenstra S, Vanmolkot F, Neef C, Burger D, Maliepaard M. Investigation into the interchangeability of generic formulations using immunosuppressants and a broad selection of medicines. Eur J Clin Pharmacol. 2015;71(8):979-90.
15. World Health Organization. WHO Expert Committee on Pharmaceutical Preparations. Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. in WHO Technical Report Series, No. 992. 2015, Annex 7 [homepage on the Internet]. [cited 2016 Apr 11]. Available from: http://apps.who.int/medicinedocs/documents/s21898en/s21898en.pdf
16. European Medicines Agency. Committee on Medicinal Products for Human Use (CHMP). Guideline on the investigation of bioequivalence. 20 January 2010. CPMP/EWP/QWP/1401/98 Rev. 1/ Corr ** [homepage on the Internet]. [cited 2016 Apr 11]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf
17. U.S. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for industry. Bioavailability and Bioequivalence studies for orally administered drug products — general considerations. March 2003 homepage on the Internet]. [cited 2016 Apr 11]. Available from: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf
18. Gwaza L, Gordon J, Potthast H, Welink J, Leufkens H, Stahl M, et al. Influence of point estimates and study power of bioequivalence studies on establishing bioequivalence between generics by adjusted indirect comparisons. Eur J Clin Pharmacol. 2015; 71(9):1083-9.
|
Author for correspondence: Alfredo García-Arieta, PhD, División de Farmacología y Evaluación Clínica, Subdirección General de Medicamentos de Uso Humano, Agencia Española de Medicamentos y Productos Sanitarios, 1 Calle Campezo, Edificio 8, Planta 2 Oeste, ES-28022 Madrid, Spain |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2016 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source URL: https://gabi-journal.net/assessment-of-the-interchangeability-between-generics.html
Copyright ©2025 GaBI Journal unless otherwise noted.