Biosimilars in oncology: beyond regulation—building confidence through evidence and dialogue
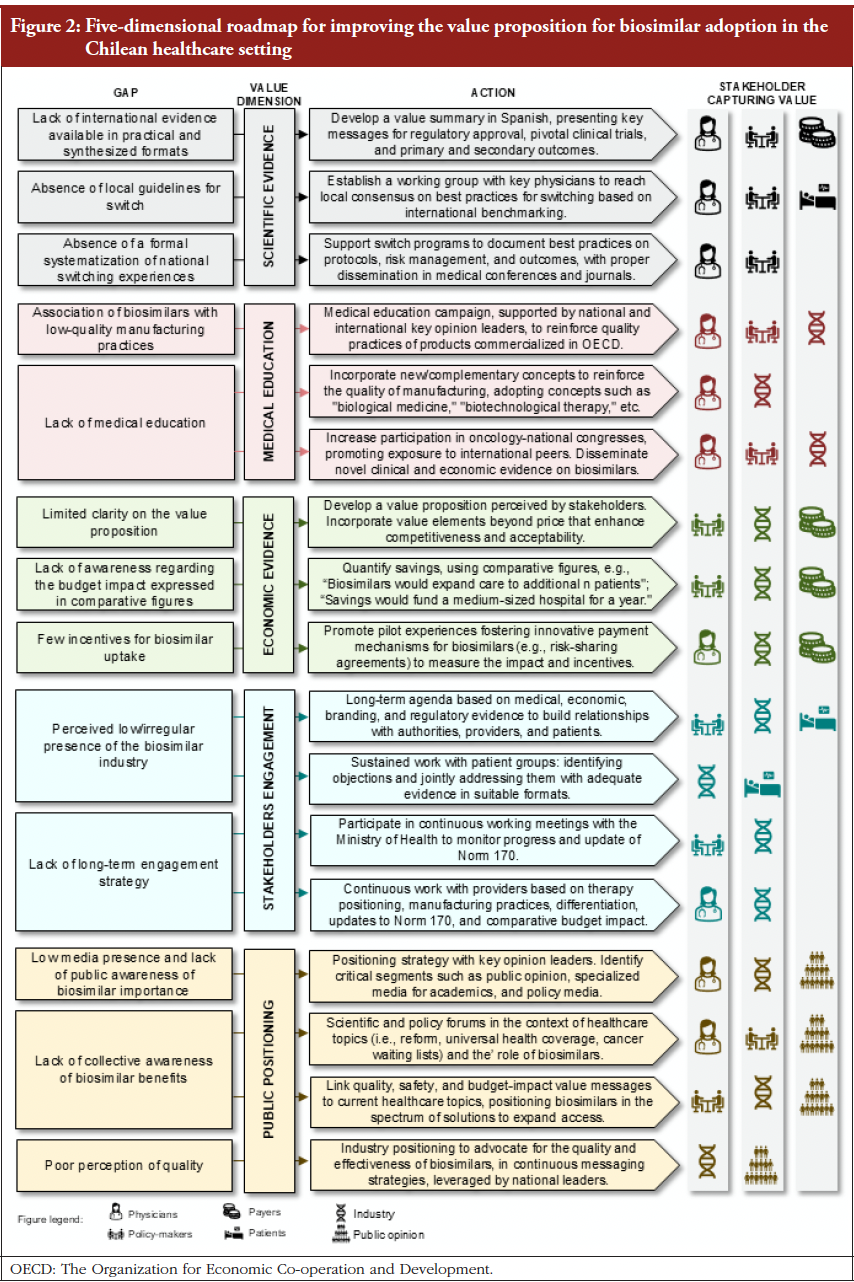
Abstract: This editorial discusses systemic barriers limiting oncology biosimilar adoption in Chile and Latin America, emphasizing trust, education, and coordinated policy actions to strengthen value, equity, and confidence in biosimilars. The manuscript by Paredes-Fernández and Lenz-Alcayaga [1] addresses a key but often neglected question in Latin America: why have biosimilars, despite their proven safety, efficacy, […]



