Generics authorization – groundbreaking regulatory approach to closer international cooperation on the rise – IGDRP
Published on 2015/01/30
Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(1):48.
Submitted: 24 January 2015; Revised: not applicable; Accepted: 27 January 2015; Published online first: 30 January 2015
The ‘International Generic Drug Regulators Pilot’ (IGDRP) seems to be a true model for the future of authorization processes. It was first founded back in April 2012 with the aim to promote the international collaboration within authorization processes of generic medicines. Members of IGDRP are Australia, Brazil, Canada, China, Chinese Taipei, EU (European Union), Japan, Republic of Korea, Mexico, New Zealand, Russia, Singapore, South Africa, Switzerland and USA, respectively, their national competent authorities, European Directorate for the Quality of Medicines & HealthCare and World Health Organization (observer). This means that a global work sharing process regarding generics authorization applications, the regulatory and scientific assessment thereof, and finally the authorization itself is ready to start.
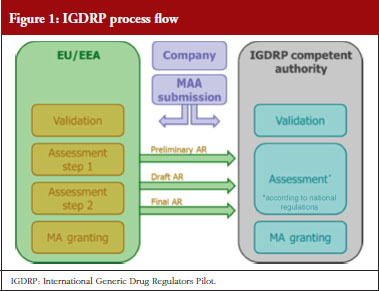
In the first phase of the pilot Australia, Canada, Chinese Taipei and Switzerland are participating and it was agreed to use the Decentralised Authorisation Procedure (DCP) of the EU as an ‘Information-sharing’ model. This means that an application for a marketing authorisation (MAA) of a generic medicinal product is filed by the applicant (criteria for eligibility, see the link in [1]) in a synchronized manner with all authorities, i.e. with the Reference Member State (RMS) and with all Concerned Member States (CMS) of the DCP within the EU, and – also at the same time – with other IGDRP-member competent authorities (external of EU). The RMS will then provide the reference scientific assessment report of such an application to all these authorities – even to the EU-externals – during the ongoing DCP procedure, see Figure 1.
With participation in the pilot phase, applicants gain the unique possibility to obtain a marketing authorization in different markets inside and outside of the EU by a coordinated process – provided that the applicant agreed to share the DCP assessment reports with some or all of the non-EU agencies. The ‘initial expression of interest’ – phase of the first pilot round is now already over and the first procedures have already started. In total about 20 requests have been submitted. The Austrian Authority – AGES Austrian Medicines and Medical Devices Agency, for example, is also participating as a selected RMS right from the beginning of IGDRP. The experience gained in the pilot phase will contribute in a valuable way to establishing best practice for the future of this exciting project. Noteworthy, it has been recently agreed by the European Medicines Agency and the IGDRP Steering Committee to also extend the Information-sharing pilot to Centrally Authorised Products (CAPs) procedures, paving the way to an even broader application of the project.
A new era of authorization of medicinal products might have begun by the start of the IGDRP project. IGDRP could indeed lead to more harmonized scientific views and better balanced generic drug assessments, not only within the EU – which was already managed by the last update of the bioequivalence guideline in 2010 – but now also on a broader international level. It should facilitate an efficient and consistent assessment procedure of generic medicinal products while reducing regulatory burden for applicants at the same time. This in fact could speed up the authorization processes to bring low priced generics even faster to the international markets to benefit health budgets.
Competing interests: None.
Provenance and peer review: Not commissioned; internally peer reviewed.
Co-author
DI Dr Katharina Gazda-Pleban, Regulatory Expert, AGES Austrian Medicines and Medical Devices Agency and Austrian Federal Office for Safety in Health Care, 5 Traisengasse, AT-1200 Vienna, Austria
References
1. Co-ordination Group for Mutual Recognition and Decentralised Procedures – Human. Information sharing pilot for the evaluation of generic drug applications involving the decentralised procedure of the European Union [homepage on the Internet]. 2015 [cited 2015 Jan 24]. Available from: http://www.hma.eu/451.html
|
Author for correspondence: Christoph Baumgärtel, MD, MSc, Senior Scientifi c Expert, Coordination-Point to Head of Agency, AGES Austrian Medicines and Medical Devices Agency and Austrian Federal Offi ce for Safety in Health Care, EMA European Expert, Vice-Chair of Austrian Prescription Commission, 5 Traisengasse, AT-1200 Vienna, Austria |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.