Medicare drug price negotiations: impact on healthcare development and patient access to medicines
Published on 2023/09/14
Generics and Biosimilars Initiative Journal (GaBI Journal). 2023;12(3):95-105
Author byline as per print journal: Michael S Reilly, Esq; Thomas R Barker, Esq, JD; Charles M Clapton, Esq; Steven J Potts, PhD, MBA; Andrew Spiegel, Esq
|
Introduction: In the US, a Medicare drug price negotiation provision has been introduced in the form of the 2022 Inflation Reduction Act (IRA). An online webinar on the introduction of the IRA was held to discuss its implications on medicines innovation and impact on patient access to medicines. |
Submitted: 8 October 2023; Revised: 6 November 2023; Accepted: 8 November 2023; Published online first: 21 November 2023
Introduction
In 2022, the Inflation Reduction Act (IRA) (Public Law 117-169) was signed into law in the US. This is designed to empower the US Secretary of Health and Human Services (HHS) and enable them to develop and implement methods and a process to negotiate a limited number of maximum fair prices (MFPs) for prescription drugs in the Medicare programme, directly with manufacturers. At present, under the negotiation programme, the US government has selected 10 Part D drugs for negotiation for initial price applicability in year 2026 [1]; and this will scale up to 20 Part B and Part D drugs by 2028 [2]. The 10 Part D drugs are: apixaban (Eliquis), empagliflozin (Jardiance), rivaroxaban (Xarelto), sitagliptin (Januvia), dapagliflozin (Farxiga), sacubitril/valsartan (Entresto), etanercept (Enbrel), ibrutinib (Imbruvica), ustekinumab (Stelara), insulin aspart (Fiasp, Fiasp FlexTouch, Fiasp PenFill, NovoLog, NovoLog FlexPen, NovoLog PenFill). However, several drugmakers have sued the US government over the IRA [3, 4].
With this context, a webinar entitled ‘Drug price negotiations: impact on healthcare development and patient access to medicines’ was held by the Alliance for Safe Biologic Medicines (ASBM) in collaboration with the Generics and Biosimilars Initiative (GaBI) on 26 July 2023.
This webinar examined how the Medicare drug price negotiation provision can be considered a form of price-setting policy, similar to the European-style price control policies applied to medicines. It also sought to gain an understanding about the perceived threat that government price setting could pose regarding access to medicines for patients, from a healthcare providers perspective. Experiences with cost containment efforts in different countries and their impact on patient care and innovation were a key focus.
Methods
In this online event, held on 26 July 2023, the contributors discussed the creation of Medicare Part D, Medicare drug price provision and MFP determination. In addition, aspects of the US Medicare drug price negotiation system were compared to the system in Europe and discussions were held on whether the US government price setting diminished access to medicines for patients and the impact of the IRA’s small molecule penalty on cancer drug innovation.
The webinar was introduced by Michael S Reilly, Esq, Executive Director of ASBM, and moderated by Steven Stranne, JD, MD, partner at Foley Hoag LLP. Expert presentations were delivered by Thomas R Barker, Esq, JD, Partner, Foley Hoag LLP, USA; Charles M Clapton, Esq, Vice President, Federal Government Affairs, Gilead Sciences, USA; Matias Olsen, Public Affairs & Policy Manager, EUCOPE, Belgium; Steven J Potts, PhD, MBA, biotech drug developer, International Cancer Advocacy Network, USA; Professor Philip J Schneider, MS, FASHP, FASPEN, FFIP, Ohio State University; former Vice President of the International Pharmaceutical Federation (FIP); and Andrew Spiegel, Esq, Executive Director, Global Colon Cancer Association (GCCA), USA.
Results
Expert speaker presentations
There were several expert speaker presentations, followed by a Q&A session and an in-depth panel discussion, moderated by Dr Steven Stranne.
Mr Reilly opened the webinar by noting that the introduction of the IRA brings several changes to Medicare Part D.
He outlined that Medicare part D is a federal programme providing outpatient prescription drug coverage for Medicare beneficiaries. It was implemented in 2006 as part of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Enrolment in Part D is voluntary, with the exception of those who qualify for both Medicare and Medicaid, who are automatically enrolled to ensure they have prescription drug coverage. He noted that there is a 90% satisfaction rate amongst beneficiaries.
It was highlighted that price ‘Negotiation’ (Price Setting) for Part D was considered and rejected. Price Setting has long been known to have a negative impact on innovation, as it reduces the potential return on investment for drug manufacturers. The webinar set out to provide historical evidence of this effect in Europe and elsewhere. Ultimately, this could lead to fewer drugs for US patients in the future, as well as for patients worldwide, given that the US is the global leader in pharmaceutical manufacturing, responsible for producing more than 60% of the world’s new drugs.
The IRA builds on policy discussions dating back to the creation of Part D itself, granting ‘negotiation’ authority to the Centers for Medicare and Medicaid Services (CMS). The webinar provided a forum for experts from various fields to examine the potential effects of this policy on drug research and development, and patient access.
Mr Reilly emphasized that the IRA focusses on the price setting and ultimately, it does not look at value and outcomes and this is likely to lead this legislation to have a lot of negative effects and the impact of the IRA on patients and the patient community is key.
The ASBM has launched a microsite, IRApatientinfo.org, which aims to be a centre of information on IRA provisions and the aspects of IRA, as well as developments that impact the patient community.
Mr Reilly concluded that a second webinar will examine the effects of several other IRA provisions, including: 1) biosimilar entry (2-year pause); and 2) ASP+8% reimbursement.
Dr Stranne added that it is key to consider how to make sure the next generation (or generations) of patients have meaningful access to innovative technology that is life saving and life enhancing. He noted that the webinar panellists would provide a wide range of perspectives on how best to tackle the challenges in establishing fair and adequate pricing for innovative pharmaceuticals and biologicals. The webinar brought together perspectives of key stakeholders including the patient and family, pharmacy representatives and regulatory advocates who have spent their entire careers focusing on patient access. In addition, there were industry perspectives as well as perspectives from both the US and Europe.
The creation of Medicare Part D and why Medicare drug price negotiation is really a particularly flawed form of European price controls and what that means for patients
This presentation was given by Mr Thomas R Barker, Esq, JD, a former Commissioner of the Medicaid and CHIP Payment and Access Commission (MACPAC), an advisory body that provides policy advice to Congress and the States on the Medicaid and CHIP programmes. He played a key role in the implementation of every major health policy initiative enacted during his time at the HHS between 2001 and 2008, including the Medicare Prescription Drug Benefit (Medicare Part D) and the Medicare Advantage programmes. He also chaired policy briefings on Medicare and Medicaid policy at the HHS, the Office of Management and Budget, and the White House.
The creation of Medicare Part D
Historical context
Mr Barker initiated the discussion by providing an introduction to the historical background of Medicare, particularly focusing on the year 2003 when the Medicare Modernization Act (MMA) was enacted.
He highlighted how the MMA introduced an alternative strategy for regulating costs and managing expenditure within the Medicare program, particularly in Part D. Additionally, Mr Barker noted how the IRA reversed the approach that had been previously embraced in the MMA for regulating prescription drug prices.
Since the enactment of the Medicare and Medicaid programmes in 1965, Congress and the Executive Branch have struggled to control healthcare spending in the Medicare and Medicaid programmes. In 1983, Congress and the Reagan Administration agreed on a new payment system for hospitals inpatient perspective payment system (IPPS) that was designed to control costs. Congress and subsequent Administrations adopted similar prospective payment models between 1987 and 1997 for other classes of providers. However, these payment models failed to meaningfully control costs. For example, Medicare payments to hospitals were US$36 billion in 1980; in 2021, they had grown to US$450 billion, an increase of 1,150%. Core inflation during that four-decade period, by contrast, was 280%. For virtually every category of Medicare services (hospitals, physicians, home health, skilled nursing, dialysis), the government strictly regulates pricing. The government sets a rate of payment and providers must accept that payment rate as a condition of participating in the Medicare programme. Mr Barker noted that the IRA uses the misleading phrase price negotiation while there is no negotiation. The Medicare payment rate is a take-it-or-leave-it price. Providers have a right to comment on proposed payment policies, but at the end of the day, the government establishes a set price for each medical procedure. Thus, the term price negotiation is a misnomer, it is going to be like every other payment system in the Medicare programme where CMS establishes the rate of payment and providers, Part D plans and pharmaceutical and biological manufacturers will be forced to accept that price given the history of price controls in the Medicare programme. CMS has final authority to set the rate of payment and providers, health plans and pharmaceutical and biological manufacturers will be forced to accept that price. Unlike every other payment system in Medicare, however, MFP gives CMS an unprecedented (and poorly defined) mandate to set the price based on a subjective ‘lowest maximum fair price’ standard that is not applied in other healthcare sectors.
Part D: a different model
Mr Barker then introduced the concept of Part D. When Congress created the Part D programme in 2003, it had the benefit of two decades of experience with healthcare provider rate setting. There was general consensus among both political parties, going back to the Clinton Administration, that if Medicare was going to adopt an outpatient prescription drug benefit, there needed to be a new model to control prices because government rate-setting had failed to control prices in all other sectors of the healthcare marketplace.
It was also highlighted that when President George W Bush signed Part D into law in December 2003, it relied on an entirely different model than the rest of Medicare:
- The benefit would be run by private insurance plans
- Negotiation for prices paid to pharmacies for drugs dispensed to Medicare beneficiaries would be conducted by pharmacy benefit managers and not the government
- The government would be prohibited from instituting a formulary of covered drugs, from negotiating drug prices and from interfering in negotiations between Part D plans, manufacturers, and pharmacies
In summary, Part D has been enormously successful at controlling costs. When Part D was enacted in 2003, Congress estimated that spending on Part D drugs during the period of 2004–2013 would be US$770 billion. Actual expenditures were 45% lower, amounting to US$421 billion. The average monthly premium for part D in 2023 is US$32. In 2006, the average monthly Part D premium was also about US$32. By contrast, the Part B premium in 2006 was US$88.50; but it is now US$165 in 2023. This shows that the private marketplace is far more effective at controlling costs for the government and for beneficiaries. Notably, the Part B drug payment programme has successfully relied on a competitive market-based price to balance access and affordability, which has kept Part B drug inflation in check.
The IRA drug price ‘negotiation’ process will not be a negotiation at all
Mr Barker noted that the so-called IRA negotiation process will simply substitute government rate setting with private market negotiations that have been enormously successful in limiting premiums and Part D expenditures. In that sense, the process is simply the European version of price controls. Government rate-setting of Medicare payments for other services has not been successful in controlling Medicare spending over the past four decades. Consequently, there is no reason to believe that a government-run rate-setting process will be at all successful in lowering programme costs. The Part D benefit redesign that was included in the IRA will provide benefits for enrollees, but those benefits have nothing to do with the drug price negotiation provisions of the law.
Why Medicare drug price negotiation is really European price controls and what that means for patients
In his second presentation, Mr Barker discussed the effect of Medicare drug price negotiation on patients and other factors to increase the Part D spending.
1. Reductions in innovation
There is going to be a reduction in the innovation of medicines. Manufacturers will be less likely to invest in new breakthrough therapies because the loss of profit incentives, which is built into the system, will go away under the IRA drug pricing policies.
More alarming, some members of Congress are proposing to apply the IRA’s government price-setting policies to launch prices of innovative products. This will further stifle innovation.
2. Effect on premiums
Although it seems counter-intuitive, the price-setting provisions in the IRA may cause premiums to increase.
Part D premiums have been essentially flat over the past 20 years, while premiums in other parts of Medicare have escalated at rates equal to or even higher than inflation.
3. Other factors at play are likely to increase Part D spending
Top-line statistics will show that Part D programme costs have increased more rapidly than in prior years by other factors. For example:
- New GLP-1 agonists are going to drive programme spending. Here, the drugs are now being used for weight loss and physicians are prescribing them for haemoglobin A1c (HbA1c) and diabetes control, which will drive up Part D spending
- New Alzheimer’s agents (especially if reformulated as Part D drugs) are going to drive programme spending
- New gene and cell therapies (especially if reformulated as Part D drugs) are going to drive programme spending
In conclusion, Mr Barker stated that government price setting in the Medicare and Medicaid programmes have historically been ineffective in controlling programme costs. The free-market approach adopted on the enactment of Part D 20 years ago has clearly demonstrated the superiority of a private market approach to control Medicare spending. He warned that government rate setting of prescription drug prices will hinder innovation and ultimately harm patients.
Introduction of Medicare drug price provision and maximum fair price (MFP) determination
This presentation was given by Mr Charles M Clapton. Mr Clapton has nearly two decades of Capitol Hill experience, Notably, he served as health policy director for the Senate Committee on Health, Education, Labor, and Pensions, aiding the passage of the US Food and Drug Administration Safety and Innovation Act (2012). He also played a pivotal role as a lead Republican staffer during the Affordable Care Act’s congressional deliberations. He impacted the House Ways and Means and Energy and Commerce Committees by shaping Medicare Part D prescription drug benefits, revise drug payment methods for Medicare Part B, and Medicaid changes in the Deficit Reduction Act of 2005.
Medicare Part D
The intent behind Part D was to create a market-based alternative to government price setting. Herein, the MMA established a programme where private plans negotiated with manufacturers and provided competing choices to patients, allowing beneficiaries to choose the plan that best met their needs. The Part D benefit was very successful, particularly in holding premiums essentially flat for almost two decades. At the same time, some beneficiaries have faced significant increases in out-of-pocket costs.
Ex-US price controls – limitations on patient access
Prior to the enactment of MMA, and at an accelerating pace since then, many other countries particularly those in the Organisation for Economic Co-operation and Development (OECD) countries have implemented significant price controls on innovative drugs, or ex-US price controls. These price controls have achieved significant discounts, often well below the prices available in the US. These price controls have also resulted in patients in those countries facing limitations on access to innovative new medicines. This includes drugs not being approved as well as coverage not being provided for significant periods of time after the same drugs are available to US patients. This unfortunate outcome for patients is a foreseeable consequence of certain price control policies that have been implemented. It is worth noting that both Democratic and Republican lawmakers share a common frustration regarding these price controls. This has also led to a dynamic where the total costs of developing innovative new medicines have been shifted, so that the US healthcare system and patients are bearing an ever-greater share of total research and development (R & D) costs across the industry.
Inflation Reduction Act
Anger over ex-US drug prices, increasing out-of-pocket (OOP) costs, coupled with support for expansion of Medicare price setting authority ultimately led to enactment of the IRA in 2022.
There are three main components of the IRA:
- Inflation penalty – this imposes a cap on the level of price increases the manufacturers can take
- Redesign Part D benefit (to address OOP issue)
- ‘Price Negotiation’ – a mechanism that would allow the government to negotiate prices
Generally, negotiation is a misnomer, it is really government price setting. Medicare will directly negotiate an MFP for a subset of brand drugs without generics or biosimilars competition. Negotiation will occur nine years after launch for small molecule drugs, 13 years for biologicals.
The bill’s creators picked these arbitrary dates of 9 and 13, and the reasoning behind their selection is not clear. However, this choice has potentially resulted in unintended consequences, which have tangible effects on the future of drug development. This brings us to the topic of negotiation mechanisms.
In 2026, the first year of price negotiation, 10 Part D drugs will be subject to negotiation. By 2030, this will have expanded to include 80 drugs subject to negotiation, covering both Part D, which includes small tablet drugs from a pharmacy, as well as Part B, which primarily consists of physician-administered injectables. Penalties for refusal to accept the ‘negotiated’ price are significant tax penalties for manufacturers, which, in fact, are excise taxes that can rise up to as much as 95% of total revenues of the drug. This essentially compels any manufacturer to participate in this negotiated price mechanism. This brings us back to why ‘negotiation’ is a misnomer, considering the concept of negotiation.
Negotiation process
The negotiation process is a misnomer. What is going to happen is that manufacturers will be required to submit several specified types of data.
CMS will consider the following data and then make determinations as to what they think the price of the drug should be:
- research and development costs and the recoupment of those costs
- unit costs of production
- distribution costs
- prior federal financial support for novel therapeutic discovery and development, e.g. did National Institutes of Health (NIH) make any investments or were there other federal grants involved in the development of medicine?
- approved and pending patent applications
- Food and Drug Administration (FDA)-recognized exclusivities, and certain other applications and approvals
- market data, and revenue and sales volume data
The negotiation process has minimal requirements for patient engagement, with no formal stipulations mandating the inclusion of patient voice. This stands in stark contrast to the patient engagement and process requirements seen in European Health Technology Assessment (HTA) programmes.
Negotiation is not required to go through rulemaking, it is conducted through sub-regulatory guidance and is exempt from judicial review. Therefore, even if it appears arbitrary or in violation of basic due process requirements, unless there is a constitutional claim, judicial review is precluded. This is in sharp contradiction to the implementation of the original Part D benefit, where interested parties could submit comments, and those comments had to be considered in the development of the new law.
Impact and consequences
The Congressional Budget Office estimated the IRA will save US$237 billion over 10 years. There is significant uncertainty on the levels of discounts that negotiation will result in. Several reports have already highlighted the likely potential impact the IRA will have on future drug development. Vital Transformations, which is a research-based organization, worked very closely with trade organization BIO and PhRMA, recently issued a report that found that the IRA price controls will result in as many as 139 drugs over the next 10 years not being developed at all [5]. Academic researcher Tomas Phillipson at the University of Chicago, who served in a senior role during the prior administration in the White House, conducted research that showed similar negative impacts on development of new drugs [6]. The No Patient Left Behind Coalition has shared analyses highlighting the concerns of venture capital investors showing how the IRA is discouraging investments in certain types of drugs [7].
In conclusion, these analyses indicate that the IRA will disproportionately discourage future investments in and development of certain types of drugs:
- Small molecule drugs – manufacturers will begin shifting their focus away from making future investments in small molecule drugs and instead, they will tend to invest in areas like biologicals, primarily due to the extended negotiation periods available
- Subsequent indications for already approved drugs, for example, Keytruda, along with many in the field of cancer oncology, typically follow a model where they begin with a small patient group in a high level of unmet medical need. After gaining approval, they expand to earlier stages of treatment or larger indications and patient population. This approach is currently rewarded under the Hatch-Waxman Act, which offers extended exclusivity for new clinical trials and indications. However, under the IRA, there is a hard cap. For a small molecule drug treating glioblastoma, a nine-year countdown begins. Consequently, manufacturers will be disincentivized from conducting additional clinical trials to explore its potential efficacy against other cancer types
- Rare diseases treatments.
Looking forward, the Biden Administration has already included a proposal in its FY 2024 Budget to accelerate price negotiation to apply to drugs at five years after launch and it would also increase the number of drugs subject to negotiation. Twenty-eight Senate Democrats have already co-sponsored the SMART Act which also accelerates negotiation to five years. In the longer run, federal budgetary pressures will insentivize Congress to expand price negotiation to apply at launch, using the mechanisms established by the IRA. This would further exacerbate the negative and unintended consequences on drug development.
Measuring the damage: impact of the IRA small molecule penalty on cancer drug development
Dr Steven J Potts, a successful biotech entrepreneur, discussed the impact of the IRA’s small molecule penalty on cancer drug innovation. Dr Potts has over 20 years of experience in the field of cancer, specializing in small molecules, complex cell therapies and biologicals. He contributed to the development of two approved drugs and supported molecular testing for entrectinib in 15 countries with 30,000 patients, a drug that obtained breakthrough designation equivalents in the USA, Europe and Japan.
Dr Potts’ presentation focused on the current effects of the IRA and how these effects (the level of damage) can be measured going forward. He highlighted that IRA imposes Medicare price controls on small molecules drugs at nine years after launch and on biologicals at 13 years, there seems to be no logical reasoning behind this difference. However, this distinction significantly impacts the ability of drug developer to secure funding from venture capitalists for small molecule development.
He also discussed how overall the IRA legislation reduces incentives for small molecules development compared with biologicals, because it only affects drugs purchased by Medicare [8-9], it also reduces incentives for drugs that treat seniors [10].
To better understand the current effects of the IRA, in March 2023, Dr Potts surveyed approximately 100 Venture funds in the pharma or biotech industry, asking two main questions, see Figure 1 [10]:
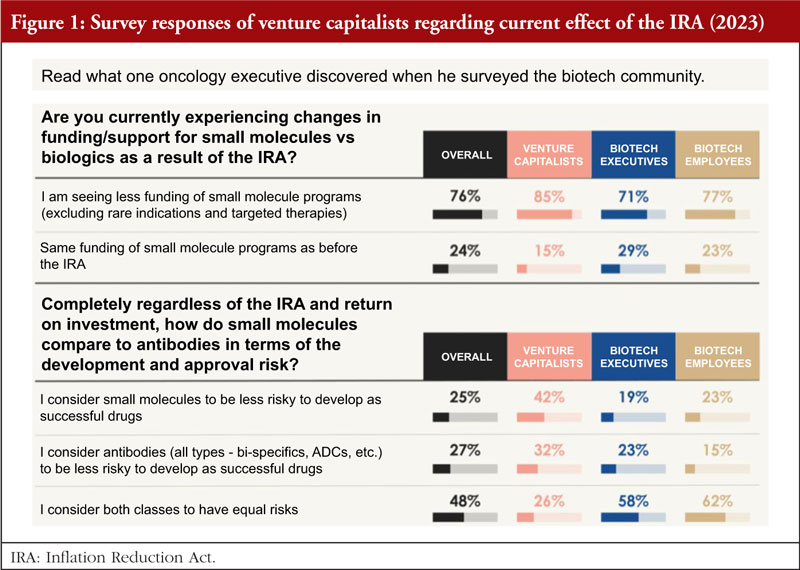
1. Are you currently experiencing changes in funding/support for small molecules versus biologics as a result of the IRA?
Eighty-five per cent said yes, they are seeing a significant decrease in interest in funding small molecules for large populations as a direct result of the IRA. This has a huge impact on fund raising for small start-up development in small molecules.
2. Regardless of the IRA and return on investment, how do small molecules compare to antibodies in terms of the development and approval risk?
Based on the survey results from March 2023, it is clear that both classes of drugs were considered to carry similar risks overall, 42% consider small molecules to be less risky to develop a successful drug, while 32% for antibodies; and 26% consider both classes of drug have equal risks.
The survey shows that six out of seven venture capitalists (VCs) have either withdrawn or been affected by the IRA, investment in small molecule drug development is shrinking and the vast majority of venture investors surveyed have moved away from funding small molecule programmes for Medicare patient populations as a result of the IRA.
In a similar survey on the impact of the IRA carried out by BioCentury with the support of BIO, see Table 1 [11], which was published around the same time as the Potts’ Survey of Venture Funds 2023 [10]. Key survey findings are:

- The IRA is having a broad impact on the biopharma industry
- Competitive landscapes will change, as companies are upending their pipeline and commercialization strategies
- Expect pivotal changes in orphan drug strategies and modality choice
- The IRA may accentuate the buyers’ market for partnering and mergers and acquisitions (M&A)
Sixty-six per cent of the respondents find the impact of the IRA ranged from existential crisis to major or minor changes, companies with revenues over US$1 billion see major or minor changes ahead; nearly one quarter (24%) are planning to prioritize their pipelines towards biologicals. All but one of these are US-based companies, 29% are weighing-up whether to shift to biologicals.
The IRA is particularly devastating in the neuroscience field, where timelines for development are already longer, success metrics lower and reimbursements not at all assured. The IRA will drive choice to invest in non-Medicare indications.
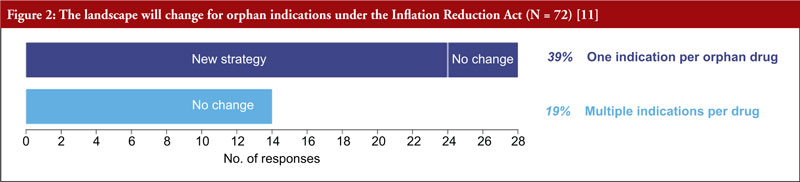
As one orphan indication per drug is the default, patients and companies may not get the most value possible from each drug. Because of provisions in the IRA, more than one third of the respondents will now prioritize orphan diseases over common, pursue one orphan indication instead of multiple, and develop distinct products for the indications, see Figure 2.
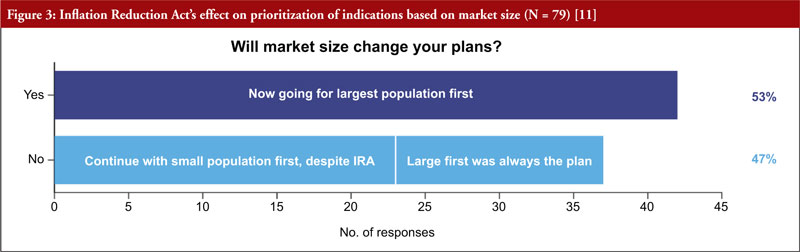
It was also clear that starting with small indications will fall out of favour, as more than half of respondents (53%) said they would go to market first in the largest population, see Figure 3. More than two thirds (68%) of respondents will rethink how to go about adding indications for approved drugs. Almost half (47%) of respondents are considering a new geographical strategy, considering to first commercialize their products in smaller indications abroad, and keeping large ones stateside.
Key points regarding the impact of the IRA on the pipeline strategy in the BioCentury 2023 survey:
- Under the IRA, products will be subject to price setting nine years after the approval of new drug applications (NDAs) and 13 years after approval of biologics license applications (BLAs).
- As a result, companies may be incentivized to seek approval in the broadest possible indication first to maximize total sales in the time before they become subject to price setting.
- It may also discourage conducting trials to add new indications late in the window before price setting.
- Orphan drugs are exempt from price setting but only if approved for a single orphan indication. This means approval of another indication can make the medicine subject to price negotiation.
- The survey asked how these changes might affect respondents’ pipeline strategies, including their selection of indications and modalities.
Dr Potts asked, ‘Will ‘pipeline-in-a-product’ become a thing of the past?’, regarding this, the BioCentury data show that most are recalibrating the calculus for adding indications, see Figure 4.
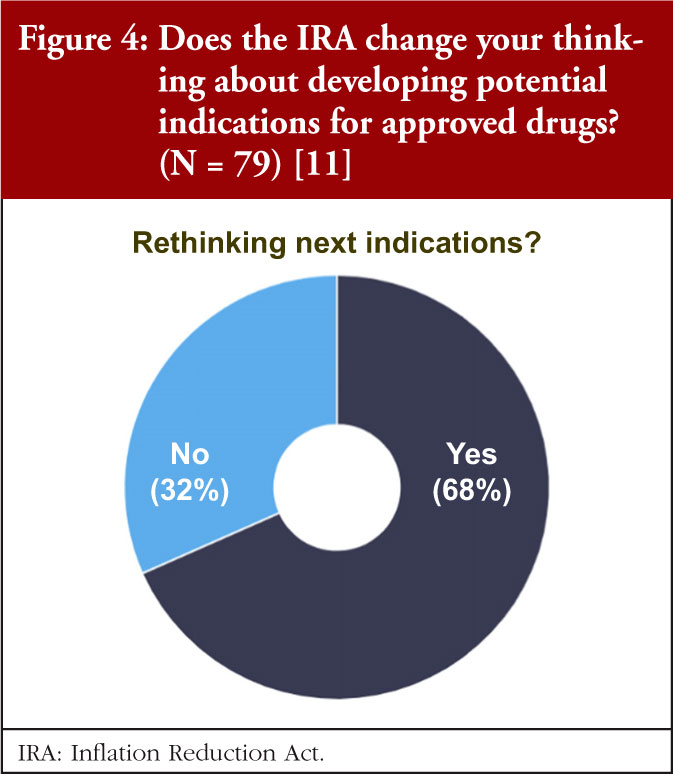
More than two thirds of respondents say they will rethink how they go about adding indications for approved drugs. Parallel development will likely be the best way to target multiple indications in the allotted time window. For others, it may mean building a timeline of different indications from a single product is no longer commercially viable.
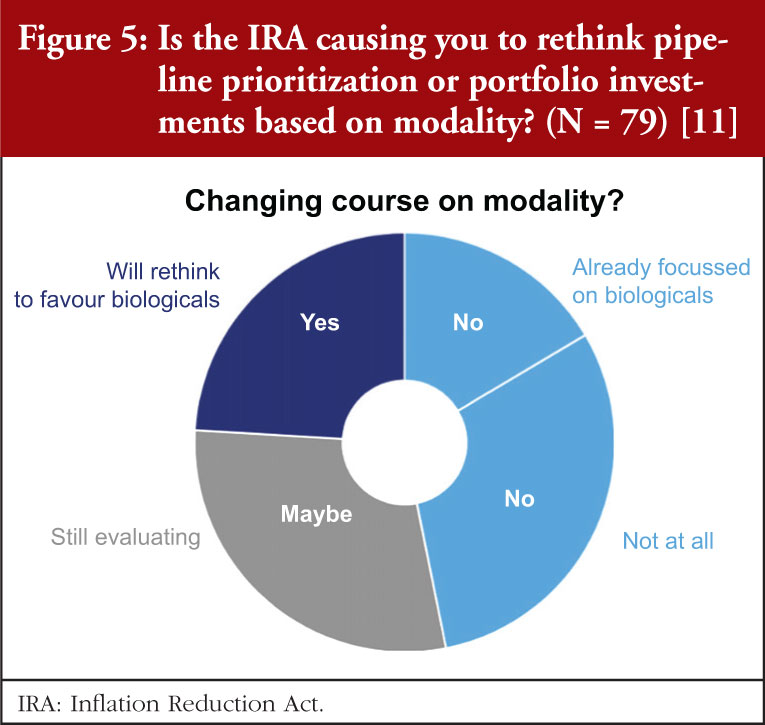
The survey data also indicate that we can expect to see a shift from small molecules to biologicals, see Figure 5. Nearly a quarter (24%) indicated that they were planning to prioritize their pipelines towards biologicals. All but one of these companies were US-based. Another 29% are weighing-up whether to shift to biologicals. A sizable minority (38%) have no plans to rethink their pipelines, with the balance (16%) already focused on those modalities.
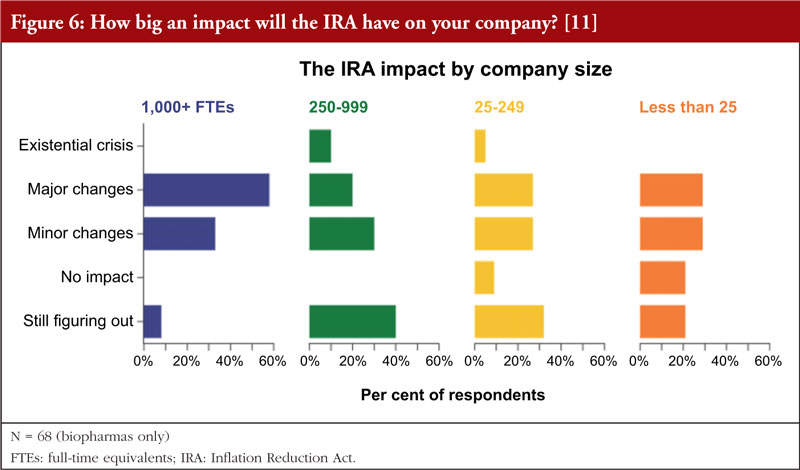
In addition, the data indicate that the smaller the company, the less relevant it sees the impact of the IRA, see Figure 6. The few companies that do not expect any impact were in the small to very small categories (<250 FTEs (full-time equivalents)). However, even among this group the majority (55%&ndash60%) expect major or minor changes. While 40% of mid-size companies (250&ndash999 FTEs) are still figuring things out the IRA impact, but the larger companies (58%) expect major changes.
In conclusion, these initial survey data and studies are ‘canaries in the coal mine’. Officials are requesting data on the impact of the IRA discouraging innovation in medicines, and they plead for further and more studies with data measuring the impact, challenges, and adverse effects on new drugs that may not be developed over the next six to 10 years because of the IRA shift unless it gets changed, particularly for small molecule drugs.
Cancer and neurological treatments, including Alzheimer’s disease with smaller indications, are going to be hit hardest by the IRA. It is possible that entire classes of innovation will be eliminated unless it is modified to grant both small molecule and biological classes 13 years prior to price setting. This is because it takes a long time for patients to change their medication, and no matter how good the drug is, sales always ramp up late. Therefore, those last four years will provide half of the profits.
Dr Potts provided resources of two revealing interviews with Dr Rafael Fonseca (Multiple Myeloma, Arizona) [12] and Dr Barbara McAneny (Solid tumour cancers, New Mexico, past President, American Medical Association (AMA) [13] on the impact of IRA on oncology patient care.
Government price setting diminished access to medicines for patients
Speaker Mr Andrew Spiegel, founder of the Global Colon Cancer Association (GCCA), an international patient advocacy organization representing colorectal cancer groups worldwide. He has over 20 years of experience in patient advocacy. Beyond colon cancer, Mr Spiegel advocates for healthcare policies globally. He chaired the fundraising committee for the International Alliance of Patients’ Organizations (IAPO) and co-founded the World Patients Alliance, the world’s largest patient organization. He is a founding member of ASBM.
In his presentation, Mr Spiegel highlighted that colorectal cancer is on the rise. The rate of colorectal cancer in Americans under 55 has nearly doubled since the 1990s. The rates among people aged under 40 are predicted to double by 2030. The American Cancer Society says there are likely to be around 153,000 colorectal cancer cases detected in 2023. It is already the second leading cause of all cancer deaths, behind only lung cancer, thus more and better treatments are needed urgently.
Unfortunately, the new drug pricing rules will harm cancer drug development. Under the IRA, Medicare will be able to ‘negotiate’ with drugmakers for lower prices on an expanding list of brand-name medications. Two types of drugs are affected, both are critical to cancer treatment, see Figure 7.

The IRA problem is that there is a ‘small molecule penalty’. As was previously pointed out by Dr Potts, the IRA makes small molecule drugs eligible for price controls nine years after FDA approval – four years sooner than biologicals. Those four additional years are critical for investors when deciding whether to invest in drug development. At present, it takes an average of more than US$1 billion and more than a decade to bring a new drug to market. Half of a new drug’s value is realized between its ninth and 13th years on the market, since less than 8% of drugs in clinical trials are approved, investors must ensure their rare successes pay off.
When it comes to the shift away from small molecule investment, this IRA policy will have serious consequences for cancer treatment. AstraZeneca, Merck and other biotech companies have already raised an alarm about how investment priorities will shift away from small molecule research in the coming years. Small molecule drugs happen to be the only way to target certain cancers as large molecule biologicals are too big to penetrate cell walls and attack cancers with intracellular targets. Mr Spiegel noted, ‘It’s not as if researchers always have a choice of which type of drug to focus on’.
In terms of the ‘negotiation’ aspects of the IRA, the process is to force manufacturers to ‘agree’ to a government dictated price, the IRA imposes an escalating ‘excise tax’ that begins at 186% of a medicine’s total sales revenue and reaches a maximum of 1,900%. As was brought into focus by previous speakers, this is essentially just price setting. During the process there is only one opportunity for patients and providers to weigh in, and it is only in writing, using a bureaucratic form with word limits that must be submitted shortly after selected medicines are announced. Key implementation decisions are also exempt from public ‘notice-and-comment’ procedures and from judicial review.
It is evident that government price setting hurts innovation. An example of a European case study is reduced drug development. In the 1970s, European companies developed most new drugs. However, since the implementation of price controls in Europe, 60% of new drugs are currently developed in the US, compared to 13% in Switzerland, 8% in the UK, and 6% in Germany and France [14].
In addition, price controls reduce patient access to cancer treatment. Of cancer medicines launched globally between 2011 and 2019, more than 96% are available to US patients. Only 65% are available in other developed nations such as Australia, Japan and the UK, which employ government price-setting policy. Mr Spiegel gave the example that Australian patients have lost access to drugs due to price controls in excess of what was agreed to by government [15]. This resulted in:
- Fewer choices, not more choices for patients and physicians
- De facto forced substitution as there are no available listed products other than the government-preferred product. Example: stage IV cancer patients now being forced switched
- Concerns with long-term sustainability, i.e. reduced competition, product launches cancelled
‘Is America ready for European-style health outcomes?’, asked Mr Spiegel asked Mr Spiegel. Of new cancer medications, 90% are available to US patients within the first year of launch, whereas less than half of these are available to cancer patients in Canada, France, Germany and the UK [16]. Furthermore, cancer death rates per 100,000 are 1.6 to 1.8 times higher in Europe than those in the US [17]. If the US had European cancer death rates, it would mean more than 400,000 additional deaths each year from cancer. That would be equivalent to losing an entire city like Minneapolis, Oakland, New Orleans, or Tulsa – every year to new cancer death.
It is critical that patient communities stand up for innovation. For example, they can do this through education: Op-eds by patient advocates [18], outlines some good data on why the IRA is unfavourable for patients, and ASBM microsite: IRAPatientInfo.org – a centre of information on IRA provisions and the aspects of IRA, as well as developments that impact the patient community; and through legal challenges: Global Colon Cancer Association (GCCA) has joined a multi-stakeholder lawsuit with the National Infusion Center Association (NICA) and the Pharmaceutical Research and Manufacturers Association (PhRMA) representing the patient voice challenging the law [19].
Additional presentations were given by Matias Olsen, ‘Negotiating drug prices in the US-lessons from Europe’; and Professor Philip Schneider ‘Pharmacist perspective on the impact of drug price controls’.
Summary of panel discussions/Q&A
Following the presentations, the panel members discussed a number of questions. These discussions were moderated by Dr Stranne and are summarized here.
Question 1: How can the IRA be modified or fixed? In other words, what steps should be taken to improve existing policies to better represent the best interests of patients?
In response, Mr Spiegel noted that it is hard to fix something once it has passed into law, and it would have been so much easier for the administration to listen to all stakeholders before the IRA was implemented. However, as it stands, what needs to be fixed is anything that stifles innovation, whether it is the number of years of protection or simply getting rid of the whole negotiation idea. The concept that one can actually negotiate with the US government must be discarded because, for one, it is not a negotiation, it lacks transparency and is misleading.
The lack of transparency in the IRA is a significant issue. There has been no way for patients and patient voices to become involved in the policy discussion, and there is no transparency about how these prices are going to be negotiated. The IRA is likely to result in measurable pullback from the industry, which will mean fewer drugs for patients. There needs to be an open way for patients to get involved and have a voice, but currently, it does not seem their viewpoints will be taken into account. This lack of transparency and the absence of patient/public input are why GCCA filed the lawsuit against the administration, along with other stakeholders.
Mr Reilly noted that the administration chose to go through the guidance process instead of the ‘notice-and-comment’ process with the changes to Medicare Part D and the IRA. In general, any major change in law within HHS would go through a normal 90-day ‘notice-and-comment’ process where it is necessary to seek input from the public and patient community, the industry and the provider community. This is necessary to try to work as partners to resolve issues that are likely to be contemplated by those who are being impacted by the law. However, when it came to the IRA, this was handled through guidance which is essentially 30 days and there is no requirement to listen to stakeholders. This demonstrates that the administration was not really open to getting input from stakeholders, going forward this is a major problem just in terms of how the IRA is perceived.
Mr Barker added that in 2004/2005, the US Department of HHS consulted extensively with the public during the implementation of the new prescription drug benefits. This included a notice and comment process involving patients, pharmacy benefit managers (PBMs), and manufacturers partnered to ensure the design of the benefit was correct. However, with the IRA, it seems the government does not want input from the public with their own fixed idea of how to do it and have deliberately eliminated the public noticing comment process.
Mr Clapton agreed that the lack of the comment process was deliberate as the government wanted to be able to execute the price controls as quickly as possible.
Dr Potts added that the nine vs 13 years really are affecting the R & D of small molecules for large populations and hopes to extend the period from nine to 13 years.
Mr Reilly believes that we should view this as the beginning of a troublesome law that could worsen over time. He pointed out that in a Commonwealth Fund podcast [20], it was mentioned that we need to exercise caution and establish some guardrails because we are uncertain about the potential extent of its impact on innovation. This issue revolves around an investment dispute between the industry and the Congressional Budget Office (CBO) regarding the number of new drugs that might be affected. It is crucial to engage with investors and industry experts since they are most knowledgeable about the likely impact. While they may not have precise information, they understand the need to protect themselves and adjust their behaviour accordingly. Ultimately, the outlook is grim, with a significant number of molecules and various indications expected to be adversely affected. Even those who support the law acknowledge that innovation and R & D will be negatively affected.
Question 2: Should we experiment with the IRA drug price negotiation approach to assess its potential in price control? If innovation is undermined, maybe we can find an alternative solution.
Dr Potts noted that there are some great small molecules for lung cancer treatment. If we go back to year 2000, a drug called Tarceva, which was the very first epidermal growth factor receptor (EGFR) small molecule, was good but not great and had lots of side effects. However, from this, there was a staircase of investment and development that has led to continually better drugs for lung cancer treatment over the last 20 years. Some now have almost no side effects and allow for a great quality of life and longer life expectancies. We are also seeing similar staircases with pancreatic cancer, colon cancer and ‘melanoma drugs. However, what this IRA approach is saying is, lets eliminate these staircases and experiment with what might happen.
Mr Reilly added that Dr Potts had an interview conversation with Drs Fonseca and McAneny [12, 13], during which they discussed the system’s current state in terms of its research capabilities, highlighting that it is at its peak. The advantages in the US system before the implementation of IRA are recognized by Europe. Both the patient and provider communities were also in favour of the old system. Policymakers are struggling to make a logical argument for the benefits of the IRA system, which was proposed and pushed forward with little public input, as a system that has any benefits other than cost savings. The question remains: where do these cost savings accrue, and how do they manifest?
Dr Potts argued that when you consider a condition such as bladder cancer, the costs associated with the disease become substantial when the bladder has to be removed, along with the expenses for the drugs required afterwards. However, if this condition can be prevented with the development of a drug, these costs can be avoided. Unless you take into account the totality of what the drug is accomplishing, you cannot really quantify any savings.
Mr Clapton voiced his concern that there is a broader issue related to cost. From a legislative lawmaker perspective, he agrees with the long-term value that medicines offer, particularly either transformative or even curative therapies. However, unfortunately, the framework that the CBO uses is very narrow, primarily looking at short-term expenditures and not taking into account the long-term benefits to the healthcare system. In terms of the question, he noted that he is particularly concerned about the IRA. As the IRA price control mechanism moves forward, the baseline assumption for CBO is that more drugs are subject to negotiation, resulting in more saving for the federal government. This will make it increasingly challenging to unwind, especially when considering the fiscal challenges facing the US. For example, the Hospital Insurance trust fund which funds Medicare, is set to be introduced in a few years. The overall debt numbers are staggering, and this situation is only going to worsen, putting immense pressure on federal lawmakers to reduce spending. He added that unfortunately, if we are talking about repealing or even mitigating IRA, there will be a cost associated with it. And with each passing year, that cost will grow larger, making it increasingly difficult to repeal.
Question 3: How do we see the inter-relationship between the European marketplace and the US marketplace?
Dr Olsen commented that in Europe, there have been changes and increased pressures, particularly on developers of innovative drugs. These pressures are now also present in the US, which does not bode well for innovation. In Europe, the World Health Organization regional office set up a stakeholder consultation called the ‘Access to novel medicines platform’, where discussions with governments took place. The idea of wealthier countries contributing more money for development was a topic of discussion. The pressures are quite apparent, and the concept of cost-sharing is conceivable.
Conclusions
The webinar provided important insight on key elements of the IRA’s Medicare drug price negotiation provisions, and the concerns surrounding their modification of successful, market-based models for delivering prescription medicines via a medical benefit (Medicare Part B) and pharmacy benefit involving competing prescription drug plans (Medicare Part D) that are strongly supported by beneficiaries.
Several fundamental flaws with the IRA’s drug pricing provisions surfaced, both in the construct of the statute and the approach CMS has taken to implementing it via sub-regulatory guidance. These flaws make clear that, despite its name, the programme is a form of government price setting, not negotiation, At the same time, the webinar brought to light several distinct flaws within the drug price negotiation programme that are not found even in European systems and are likely to worsen the harm done by the law in regards to continued progress and patient access to treatments and local physicians. These include extreme penalties for manufacturers failing to agree to negotiate, a basic lack of clarity and predictability in evidence standards and decision-making by CMS, lack of an open and transparent process by which patients and physicians can provide meaningful input, and a prohibition against appeals and judicial review.
In-depth discussions were held regarding the likelihood that the reforms would be unlikely to contain costs and would have a negative impact on innovation and patient access to new medicines. The role of European price setting was suggested as playing a role in the disparity of patient access and health outcomes between US patients and European patients. Due to the IRA’s ‘small molecule penalty’ and its initial application only in Medicare Part D, the statute’s damaging effects are most likely to be felt earliest and most acutely among US patients with cancer, chronic conditions like diabetes, cardiovascular disease, and immune-related conditions, and rare disease patients who are relying on current and future advances in small molecule medicines.
Acknowledgement
The Generics and Biosimilars Initiative (GaBI) wishes to thank all speakers and the moderator in delivering the presentations, implementing the panel discussion, and clarifying information when finalizing the meeting report, as well as Mr Michael S Reilly for his strong support through the offering of advice and information during the preparation of the webinar.
The authors would like to acknowledge the help of the webinar speaker faculty and all participants, each of whom contributed to the success of the webinar and the content of this report, as well as the support of the moderator in facilitating meaningful discussion during the panel discussions and contributing to the finalization of this meeting report.
Lastly, the authors wish to thank Ms Alice Rolandini Jensen, GaBI Journal ;Editor, in preparing and finalizing this meeting report manuscript.
Speaker Faculty, Panelists and Moderator
Speakers and Panelists
Thomas R Barker, Esq, JD
Charles M Clapton, Esq
Matias Olsen
Steven J Potts, PhD, MBA
Michael S Reilly, Esq
Professor Philip J Schneider, MS, FASHP, FASPEN, FFIPAndrew Spiegel, Esq
Moderator
Steven Stranne, MD, JD
Editor’s comment
Speakers and moderator had provided feedback on the article content and panel discussion, read and commented the revised content of the manuscript, and approved the final report for publication.
Readers can watch replay of the IRA Medicare Drug Price Negotiations webinar via this link: https://youtu.be/1-JRK_mPXR0
Funding sources
The webinar was funded by ASBM.
The Alliance for Safe Biologic Medicines (ASBM) is a coalition of patient advocacy organizations, physicians, pharmacists, biopharmaceutical manufacturers, and others working to advance patient-centered health policy at the state, federal, and international level. Learn more at www.SafeBiologics.org
Competing interests: Mr Michael S Reilly, Esq is the Executive Director and employed by Alliance for Safe Biologic Medicines. Mr Reilly served in the US Department of Health and Human Services from 2002 to 2008.
Mr Thomas R Barker is a partner at the law firm of Foley Hoag, LLP where he represents multiple pharmaceutical, biological, and medical device manufacturers before the Centers for Medicare & Medicaid Services on matters relating to Medicare and Medicaid payment and coverage of their products. Mr Barker is also on the faculty of the George Washington University Schools of Law and Public Health and Health Services, and Suffolk University School of Law. He has no conflicts to report.
Mr Charles M Clapton is the Vice President, Federal Government Affairs, Gilead Science, Inc. Mr Clapton served as a Congressional staffer from 1995 to 2012, principally working for the House Energy and Commerce, Ways and Means and Senate Health, Education, Labor and Pensions (HELP) Committees.
Dr Steven J Potts is a consultant to a number of biotech drug development companies and serves on several biotech company boards as well as a volunteer position with the International Cancer Advocacy Network (ICAN).
Mr Andrew Spiegel is a patient advocate for the Global Colon Cancer Association as well as the World Patients Alliance. Both organizations receive funding from the pharmaceutical industry. Mr Spiegel also occasionally serves on advisory boards for the industry for which he receives some compensation. There are no conflicts of interest to report.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Michael S Reilly, Esq
Thomas R Barker, Esq, JD
Charles M Clapton, Esq
Steven J Potts, PhD, MBA
Andrew Spiegel, Esq
References
1. GaBI Online – Generics and Biosimilars Initiative. First drugs for Medicare price negotiation selected [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Nov 6]. Available from: www.gabionline.net/policies-legislation/first-drugs-for-medicare-price-negotiation-selected
2. CMS.gov. Inflation reduction act and Medicare drug price negotiation [homepage on the Internet]. [cited 2023 Nov 6]. Available from: https://www.cms.gov/inflation-reduction-act-and-medicare/medicare-drug-price-negotiation
3. GaBI Online – Generics and Biosimilars Initiative. Four drugmakers sue the US government over the inflation reduction act [wwww.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Nov 6]. Available from: www.gabionline.net/pharma-news/four-drugmakers-sue-the-us-government-over-the-inflation-reduction-act
4. GaBI Online – Generics and Biosimilars Initiative. More drugmakers sue over IRA yet one withdraws [wwww.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Nov 6]. Available from: wwww.gabionline.net/policies-legislation/more-drugmakers-sue-over-ira-yet-one-withdraws
5. Gassull D, Bowen H, Schulthess D. IRA’s impact on the US Biopharma Ecosystem. Vital Transformation. 1 June 2023. Available from: https://www.bio.org/sites/default/files/2023-06/IRA%E2%80%99s%20Impact%20on%20the%20US%20Biopharma%20Ecosystem.pdf
6. University of Chicago. Philipson TJ, Durie T. The impact of HR 5376 on biopharmaceutical innovation and patient health. 29 November 2021. Available from: https://bpb-us-w2.wpmucdn.com/voices.uchicago.edu/dist/d/3128/files/2021/08/Issue-Brief-Drug-Pricing-in-HR-5376-11.30.pdf
7. No Patient Left Behind. 25 July 2022. The Honorable Chuck Schumer. Available from: https://nopatientleftbehind.docsend.com/view/e4cg7sgj6js5qenr
8. Potts S. Saving money for Medicare by abandoning new drugs for Medicare patients. Available from: https://rapport.bio/all-stories/ira-abandoning-new-cancer-drugs-for-medicare-patients
9. Potts S. Preserving the biotech social contract – we should all be pitching in. Available from: https://timmermanreport.com/2023/05/preserving-the-biotech-social-contract-we-should-all-be-pitching-in/
10. Potts S. Measuring the damage: IRAs impact on small molecule drug development. NPLB. 31 March 2023. Available from:https://www.nopatientleftbehind.org/publications/ira-impact-on-small-molecule-development
11. Fishburn CS. IRA survey: biotechs bracing for impact. BioCentury. 16 March 2023. Available from: https://www.biocentury.com/article/647205/ira-survey-biotechs-bracing-for-impact
12. Rafael F, Potts S. KOL conversation. The IRA: How public policy impacts innovation. AZBio. Available from: https://www.azbio.org/how-public-policy-impacts-innovation
13. McAneny BL, Potts S. KOL conversation. The impact of the IRA on oncology patient care. AZBio. Available from: https://www.azbio.org/kol-conversation-the-impact-of-the-ira-on-oncology-patient-care
14. Pipes S. Europe negotiates a poor vaccine rollout; Forbes, April 2021. Available from: https://www.forbes.com/sites/sallypipes/2021/04/26/europe-negotiates-a-poor-vaccine-rollout/
15. Wiggins J. Biosimilars Training Program 2023. Patient Advocate Perspective: Australia. [cited 2023 Nov 6]. Available from: https://youtu.be/HGjF8oBtzZw
16. IRA Patient Info [homepage on the Internet]. [cited 2023 Nov 6]. Available from. IRAPatientInfo.org
17. Smith: Democrat plan on drug costs will stifle innovation, San Antonio Express-News, 12 May 2021. Available from: https://www.expressnews.com/opinion/commentary/article/Smith-Democrat-plan-on-drug-costs-will-stifle-16172017.php
18. Spiegel A. Amend law to combat skyrocketing colorectal cancer rates (opinion). Reading Eagle. Available from: https://www.readingeagle.com/2023/05/13/amend-law-to-combat-skyrocketing-colorectal-cancer-rates-opinion/
19. NICA, GCCA, PhRMA Litigation Asserts Price Setting Provisions in the Inflation Reduction Act are Unconstitutional. PhRMA. 21 June 2023. Available from: https://phrma.org/resource-center/Topics/Access-to-Medicines/Release-NICA-GCCA-PhRMA-Litigation-Asserts-Price-Setting-Provisions-in-the-Inflation-Reduction-Act-are-Unconstitutional
20. Seervai S. What the Inflation Reduction Act really means for health care. The Commonwealth Fund. 9 September 2022. Available from: https://www.commonwealthfund.org/publications/podcast/2022/sep/what-inflation-reduction-act-really-means-health-care
|
Author for correspondence: Michael S Reilly, Esq, Executive Director, Alliance for Safe Biologic Medicines, PO Box 3691, Arlington, VA 22203, USA |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2023 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.


