Cost savings from use of biosimilars in rheumatology
Published on 2013/12/24
7 views

Published on 2013/12/24
7 views
Published on 2013/12/19
22 views
Published on 2013/12/17
128 views

Published on 2013/12/12
2 views
Published on 2013/12/10
7 views
Published on 2013/12/05
7 views
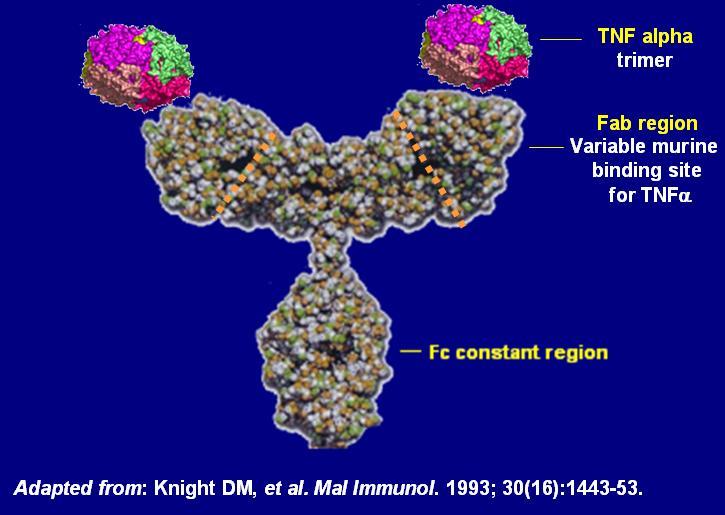
Published on 2013/12/03
18 views

Published on 2013/11/28
55 views

Published on 2013/11/26
11 views

Published on 2013/11/21
11 views

Published on 2013/11/19
67 views

Published on 2013/11/14
14 views