Patient-reported outcome measures in phase III trials of LY2963016 insulin glargine and reference insulin glargine products: ELEMENT 1 and ELEMENT 2
Published on 2017/12/15
Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(2):56-62.
Author byline as per print journal: Magaly Perez-Nieves1, MPH, PhD; Robyn K Pollom1, ANP; Ran Duan1, PhD; Samaneh Kabul1, PharmD; Amy M DeLozier1, MPH; Puneet Kaushik2, MPharm; Liza L Ilag1, MD
|
Background: This analysis evaluates the patient-reported outcomes (PROs) in two randomized studies of biosimilar LY2963016 insulin glargine (LY IGlar) and Lantus® insulin glargine (IGlar), when used in combination with mealtime insulin lispro in patients with type 1 diabetes mellitus (T1DM) or oral anti-hyperglycaemics in patients with type 2 diabetes mellitus (T2DM). |
Submitted: 24 November 2017; Revised: 4 July 2018; Accepted: 9 July 2018; Published online first: 23 July 2018
Introduction
Diabetes is a public health concern affecting an estimated 29.1 million people in the US [1] and an estimated 382 million people worldwide [2]. Insulin is a lifesaving treatment in patients with type 1 diabetes mellitus (T1DM) and is frequently used to improve glycaemic control in patients with type 2 diabetes mellitus (T2DM). In spite of the fact that 44% of diabetes patients in the US have HbA1c levels over 7%, there is only a 26% reported use of insulin. This indicates the possibility of barriers to broad acceptance of insulin with cost being considered one of the various possible limiting factors to the access of biologicals [3]. This paves the way for follow-on versions of the biological, namely biosimilar insulin with potential cost savings [4, 5].
The current evidence required to establish biosimilarity is limited to clinical measures of efficacy and safety without involving patient-reported information about their experiences [6]. Incorporating the patient perspective in the development, regulatory approval and health technology assessment of new medicines continues to gain importance to decision makers in the healthcare environment today. Patient-reported outcomes (PROs) provide a critical understanding of drug benefits and harm from the patients’ perspective and come directly from them without any evaluation by a clinician, or anyone else. They are not only an important part of a successful drug development, but also an addition to cost savings as key drivers of value proposition [7, 8]. Incorporating the patient perspective aids in gathering patient acceptability information and can serve as important drivers for the clinical decision makers [9].
Once-daily insulin glargine (IGlar, Lantus®; Sanofi US, Bridgewater, NJ, USA) is a widely used basal insulin analogue that has been shown to have a lower tendency toward hypoglycaemia compared to Neutral Protamine Hagedorn (NPH) insulin [10], and has been associated with improvements in treatment, patient satisfaction and well-being [11–13]. LY2963016 insulin glargine (LY IGlar; Eli Lilly and Company, Indianapolis, IN, USA) is an insulin glargine product with similar efficacy and safety to IGlar as evident in two phase III trials (a 52-week, open label trial in adults with T1DM; and a 24-week, double-blind trial in adults with T2DM) [14, 15]. The scope of the phase III studies was to evaluate the clinical efficacy and safety outcomes of LY IGlar compared to IGlar. A secondary objective of these clinical trials was to evaluate the patient-reported outcomes of treatment satisfaction and fear of hypoglycaemia between the two treatments.
LY IGlar is the first insulin product to be approved through an abbreviated approval pathway under the Federal Food, Drug, and Cosmetic Act, requiring submission of 505(b)(2) application, which relied in part, on the US Food and Drug Administration’s (FDA) assessment of effectiveness and safety for Lantus IGlar to support approval. The use of this pathway is for cases where the drug relied upon made its application under section 505 requiring new drug application (NDA) and has been explored by insulin, calcitonin and human growth hormone. For other follow-on biologicals, the 351(k) pathway is used which represents an abbreviated pathway for approving products shown to be biosimilar to an FDA-approved reference product [16, 17]. LY IGlar was demonstrated to be sufficiently similar to Lantus to scientifically justify reliance. LY IGlar-specific data was also furnished to FDA to establish the drug’s safety and efficacy [18].
The objective of the analysis was to evaluate patient-reported outcomes of insulin treatment satisfaction and fear of hypoglycaemia in patients on LY IGlar compared to IGlar.
Methods
Study design
Details regarding the primary efficacy and safety outcomes of ELEMENT 1 and ELEMENT 2 have been reported previously [14, 15]. Briefly, ELEMENT 1 was a phase III, randomized, open-label, non-inferiority study in adult patients with T1DM on basal-bolus insulin therapy. Patients were randomized to LY IGlar or IGlar, using a 1:1 unit conversion from pre-study insulin regimen to once daily LY IGlar or IGlar in combination with premeal insulin lispro. Treatment groups used a prefilled insulin injection device to deliver study drug; KwikPen™ for LY IGlar and insulin lispro or SoloSTAR® for the reference product IGlar. The treatment period was 24 weeks (primary endpoint) with a 28-week extension (52 weeks total) [19]. PROs were assessed at Week 2 (baseline), Week 24, and Week 52 or Early Discontiuation visit.
ELEMENT 2 was a phase III, randomized, double-blind 24-week, non-inferiority study in adult patients with T2DM on two or more oral anti-hyperglycaemic medications who were either insulin naïve or on prestudy IGlar. Patients administered insulin using syringes and covered insulin vials provided during the study to maintain the blinding of treatment [20]. PROs were assessed at Week 4 (baseline), Week 12, and Week 24 or Early Discontinuation visit.
Each patient provided informed consent. Protocols for each of the research trials were approved by the governing institutional review boards, and both trials conformed to the provisions of the Declaration of Helsinki.
Patient-reported outcomes
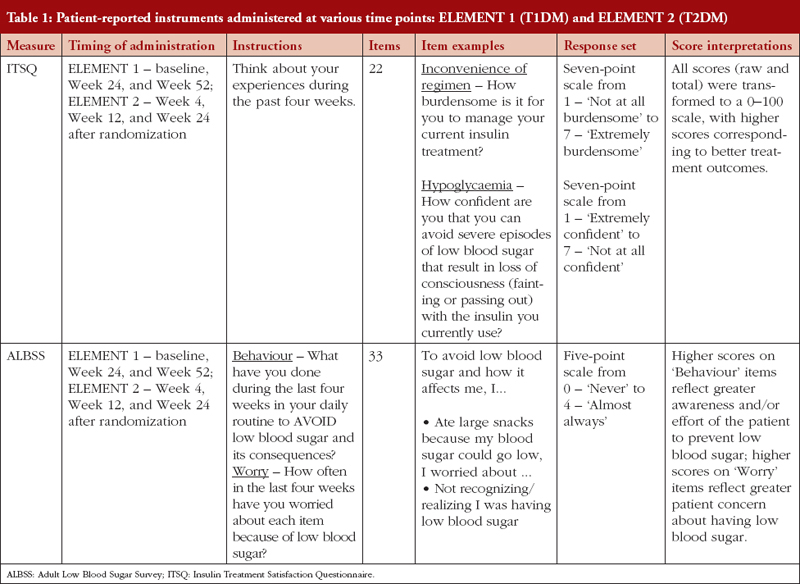
Insulin Treatment Satisfaction Questionnaire
Treatment satisfaction related to insulin therapy was assessed using the Insulin Treatment Satisfaction Questionnaire (ITSQ) [19], see Table 1. The ITSQ is a 22-item instrument with five domains: 1) inconvenience of regimen; 2) lifestyle flexibility; 3) glycaemic control; 4) hypoglycaemic control; and 5) insulin delivery device satisfaction. All raw data (domain and total) were transformed to a 0–100 scale, with higher scores corresponding to better treatment outcomes.
Adult Low Blood Sugar Survey
Fear or worry of hypoglycaemic events associated with insulin therapy and subsequent behaviours associated with avoiding future events was measured using the Adult Low Blood Sugar Survey (ALBSS), see Table 1, [20, 21]. The ALBSS is a 33-item instrument with two domains, behaviour and worry. Scores were calculated by summing patient responses to items (behaviour range 0–60; worry range 0–72). Higher scores on ‘behaviour’ items (related to avoidance of hypoglycaemia) reflect greater awareness and/or effort of the patient to prevent low blood sugar; higher scores on ‘worry’ items (related to worries about low blood sugar and its related consequences) reflect greater patient concern about having low blood sugar and related symptoms.
Statistical analysis
Demographic and baseline characteristics were summarized by treatment group for the full analysis set populations, which are based on the intent-to-treat principle and included all patients who were randomized and had taken at least one dose of study medication. The treatment groups were compared with a two-sample t-test for continuous variables and Fisher’s exact test or Pearson’s chi-square test for categorical variables. All analyses were conducted with SAS version 9.2.
All individual patient domain scores were calculated at baseline, 24 weeks, and endpoint using the non-missing items. The transformed scores (domain and total) in ITSQ and the raw scores (domain and total) in ALBSS were analysed using the analysis of covariance model for the full analysis set as specified above, including imputation of domain and total scores by last observation carried forward.
Results
Baseline characteristics
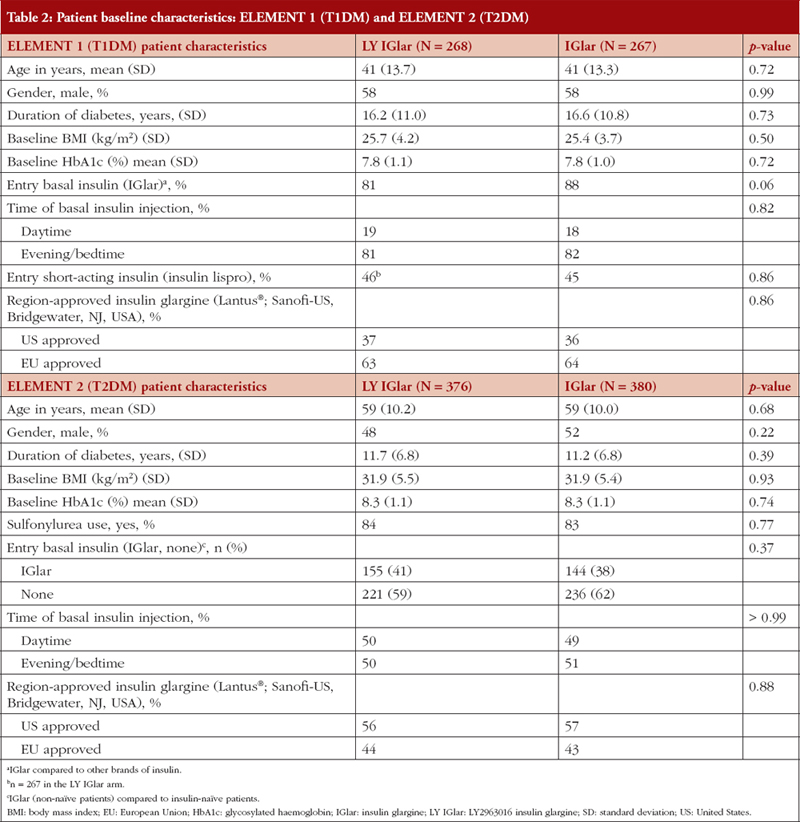
ELEMENT 1 (T1DM)
The baseline characteristics in the LY IGlar and IGlar treatment arms in ELEMENT 1 were well balanced, see Table 2. There were 535 patients with T1DM (LY IGlar, n = 268; IGlar, n = 267) with a mean age of 41 years and a mean duration of diabetes of 16.4 years. Eighty-five per cent of the patients were using insulin glargine once daily instead of Neutral Protamine Hagedorn insulin or insulin detemir before randomization (88% IGlar, 81% LY IGlar). During the study period, most patients took their basal insulin injections in the evening or at bedtime (81% LY IGlar, 82% IGlar). Some individuals did not have data collected on the ITSQ (LY IGlar, n = 4; IGlar n = 4) and ALBSS (LY IGlar, n = 3; IGlar n = 4) questionnaires at baseline or during a follow-up visit.
ELEMENT 2 (T2DM)
In ELEMENT 2, the baseline characteristics in the LY IGlar and IGlar treatment arms were well balanced, see Table 2, and included 756 patients with T2DM (LY IGlar, n = 376; IGlar, n = 380) with a mean age of 59 years and a mean duration of diabetes of 11.5 years; most patients were insulin naïve (LY IGlar 59% and IGlar 62%). In the LY IGlar treatment group, approximately 41% of the patients reported previously taking IGlar at study entry compared to 38% in the IGlar group. At Week 4 (baseline in ELEMENT 2), 7% and 9% of the individuals in the LY IGlar and IGlar treatment arms, respectively, did not take the ITSQ and ALBSS questionnaires.
Patient-reported outcomes
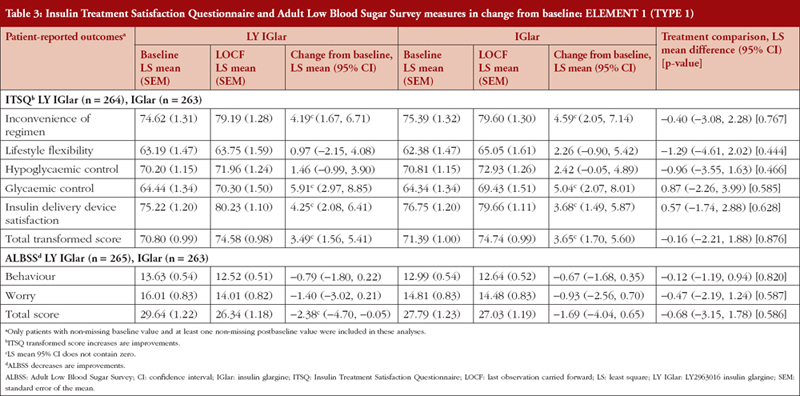
ELEMENT 1 (T1DM)
LY IGlar and IGlar treatment comparisons of ITSQ and ALBSS outcomes showed no statistically significant between treatment differences in any domains, see Table 3. In both the LY IGlar and IGlar treatment arms, there were improvements from baseline on all domains and total scores, and significant improvements on the ITSQ inconvenience of regimen, glycaemic control, and insulin delivery device satisfaction subscales, and total transformed score. Patients using the LY IGlar had significant improvements in ALBSS total score indicating reduced patient fear of hypoglycaemic events.
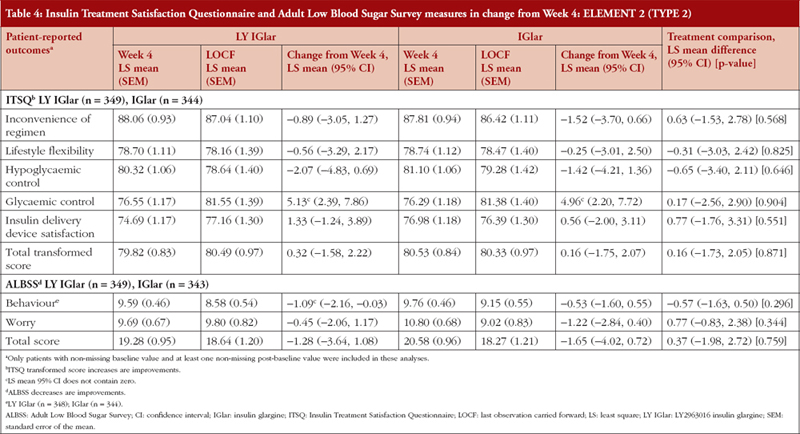
ELEMENT 2 (T2DM)
Treatment comparisons between LY IGlar and IGlar showed no statistically significant differences in change from baseline (Week 4) of the ITSQ and ALBSS total and domain scores, see Table 4. In ELEMENT 2, the ITSQ glycaemic control subscale measure for LY IGlar and IGlar demonstrated significant improvements, and inconvenience of regimen, lifestyle flexibility, and hypoglycaemic control showed a numerical worsening of scores in both treatment arms. The LY IGlar treatment arm showed significant improvements on the ALBSS behaviour subscale indicating reduced behaviours to avoid low blood sugar and its consequences.
Discussion
The findings of the studies indicate no differences in terms of PROs for biosimilar and reference insulin, signifying equal patient acceptability to use of either LY IGlar or IGlar for treatment of T1DM and T2DM. Results from clinical trials have already shown similarity in clinical efficacy and safety profiles for the two treatments in the pivotal ELEMENT 1 (NCT01421147) and ELEMENT 2 studies (NCT01421459). The PRO findings together with clinical efficacy and safety profiles demonstrate similarity between the biosimilar and reference product [14, 15].
In previous studies, IGlar was associated with greater treatment satisfaction, less fear of hypoglycaemia, and improvement in PROs when compared to therapy intensification with oral anti-diabetics or other premix insulin regimens [22, 23]. In our study, improvements from baseline on the ITSQ subscales, and total transformed score for both the LY IGlar and IGlar demonstrated patient satisfaction with the treatments. Patient satisfaction not only leads to improvement in patient’s acceptance of therapy, but may positively influence adherence and persistence which are important factors in assessing overall treatment success, in addition to the benchmark HbA1c marker [24].
Fear of hypoglycaemia has been a significant barrier for patients and physicians when deciding to start insulin therapy [25]. This can sometimes lead to delay in initiation or impact adherence/persistence of insulin, which can impact glycaemic control and lead to long-term complications with diabetes. However, in our study, where about 60% of patients were insulin-naïve, improvements in ALBSS total score indicate reduced patient fear of hypoglycaemic events in patients administered LY IGlar or IGlar. This is similar to another study comparing IGlar with targeted glulisine versus premixed insulin, which showed a less marked rise in fear of hypoglycaemia in IGlar-treated patients [26].
Further, mode of administration of drug also plays an important role in patient satisfaction. Insulin pens lead to improved patient satisfaction and adherence, greater ease of use, and improved dosing accuracy [27]. In a direct comparison in two separate clinical trials, the pen delivery devices were preferred over vial and syringe by patients [28, 29]. In ELEMENTS 1 trial, all patients used insulin pen delivery devices and experienced similar overall satisfaction with biosimilar and reference insulin.
Although these insulin therapies had similar efficacy and safety, including incidences and rates of hypoglycaemia in the pivotal ELEMENT 1 and ELEMENT 2 studies [14. 15], it is important to also look closely at the patient perspectives with consideration that these insulin glargine products are manufactured by different companies. Further, the findings should be considered cautiously as ELEMENT 1 was an open label study and there were no observed differences in hypoglycaemia rate or incidence between the two groups.
Conclusion
Patient-reported outcome measures are increasingly used alongside clinical efficacy and safety measures for the evaluation of treatment and management for diabetes. The use of this type of data is particularly important in chronic conditions where treatment can influence a person’s quality of life [30]. As expected, in our study, the patient-reported outcomes indicate similar treatment satisfaction and fear of hypoglycaemia between the two insulin glargine products: LY IGlar and IGlar. These findings along with clinical data will provide reassurance to both patients and healthcare providers that LY IGlar provides similar efficacy, safety, and patient-reported outcomes when compared to IGlar. This study further supports LY IGlar as an option for physicians and patients when choosing a basal insulin therapy that meets individual needs. The findings in this study may also help build the confidence of patients and their healthcare providers alike in use of biosimilar insulins and inform their decisions regarding treatment options for basal insulin therapy.
Acknowledgments
The authors wish to thank Emily Cullinan, PhD, and Teri Tucker, BA, from inVentiv Health Clinical (funded by Eli Lilly and Company) for their writing and editorial support.
Competing interests: This study was supported by Eli Lilly and Company and Boehringer-Ingelheim. The authors are employed by Eli Lilly and Company and/or one of its subsidiaries and minor stockholders in Eli Lilly and Company. The authors have indicated that they have no other conflicts of interest with regard to the content of this manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Magaly Perez-Nieves1, MPH, PhD
Robyn K Pollom1, ANP
Ran Duan1, PhD
Samaneh Kabul1, PharmD
Amy M DeLozier1, MPH
Puneet Kaushik2, MPharm
Liza L Ilag1, MD
1Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN 46285, USA
2Eli Lilly Services India Private Limited, Group Leader-GPORWE, Global Patient Outcomes and Real World Evidence, 1st Floor, Building Primrose (7B), Wing B, Embassy Tech Village, Devarabisanahalli, 560103 Bengaluru, India
References
1. Department of Health and Human Services. Centers for Disease Control and Prevention. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States [homepage on the Internet]. [cited 2018 Jul 4]. Available from: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
2. International Diabetes Foundation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Foundation; 2013.
3. Federal Trade Commission. Biologic drugs, biosimilars, and barriers to entry [homepage on the Internet]. [cited 2018 Jul 4]. Available from: https://www.ftc.gov/system/files/documents/public_comments/2014/02/00034-88736.pdf
4. Chow SC, Endrenyi L, Lachenbruch PA, Yang L, Chi E. Scientific factors for assessing biosimilarity and drug interchangeability of follow-on biologics. Biosimilars. 2011;1:13-26.
5. Mulcahy AW, Predmore Z, Mattke S. Perspective: the cost savings potential of biosimilar drugs in the United States. RAND. 2014.
6. U.S. Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry [homepage on the Internet]. [cited 2018 Jul 4]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf
7. U.S. Food and Drug Administration. FDA Center for Drug Evaluation and Research (CDER). Strategic plan 2013-2017 [homepage on the Internet]. [cited 2018 Jul 4]. Available from: https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM376545.pdf
8. Shivers JP, Brown AS, Yarchoan M, et al. Patient and Educator attitudes toward biosimilar insulin. Cureus. 2012;4(9):e10. [cited 2018 Jul 4]. Available from: https://www.cureus.com/posters/12-patient-and-educator-attitudes-toward-biosimilar-insulin
9. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0): What are patient-reported outcomes (17.1).
10. Clissold R, Clissold S. Insulin glargine in the management of diabetes mellitus: an evidence-based assessment of its clinical efficacy and economic value. Core Evid. 2007;2(2):89-110.
11. Barnett AH. Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag. 2006;2(1):59-67.
12. Gallen IW, Carter C. Prospective audit of the introduction of insulin glargine (lantus) into clinical practice in type 1 diabetic patients. Diabetes Care. 2003;26(12):3352-3.
13. Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabet Med. 2001;18(8):619-25.
14. Blevins TC, Dahl D, Rosenstock J, Ilag LL, Huster WJ, Zielonka JS, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015:17(8):726-33.
15. Rosenstock J, Hollander P, Bhargava A, Ilag LL, Pollom RK, Zielonka JS, et al Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015:17(8):734-41.
16. McShea M, Pollum RD. Biosimilars and follow-on biologics: a pharmacist opportunity. Pharmacy Times. 2016 Nov 16.
17. Johnson JA. FDA regulation of follow-on biologics. Congressional Research Service Report for Congress 2010.
18. U.S. Food and Drug Administration. FDA approves Basaglar, the first “follow-on” insulin glargine product to treat diabetes [homepage on the Internet]. [cited 2018 Jul 4]. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm477734.htm
19. Anderson RT, Skovlund SE, Marrero D, Levine DW, Meadows K, Brod M, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26(4):565-78.
20. Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617-21.
21. Stargardt T, Gonder-Frederick L, Krobot KJ, Alexander CM. Fear of hypoglycaemia: defining a minimum clinically important difference in patients with type 2 diabetes. Health Qual Life Outcomes. 2009;7:91.
22. Bradley C, Gilbride CJ. Improving treatment satisfaction and other patient-reported outcomes in people with type 2 diabetes: the role of once-daily insulin glargine. Diabetes Obes Metab. 2008;10(Suppl 2):50-65.
23. Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138(12):952-9.
24. Hartman I. Insulin analogs: impact on treatment success, satisfaction, quality of life, and adherence. Clin Med Res. 2008;6(2):54-67.
25. Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ. 2009;35(6):1014-22.
26. Polonsky WH, Thompson S, Wei W, Riddle MC, Chaudhari S, Jackson J, Bruno AS, Greater fear of hypoglycaemia with premixed insulin than with basal-bolus insulin glargine and glulisine: patient-reported outcomes from a 60-week randomised study . Diabetes Obes Metab. 2014;16(11):1121-7.
27. Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4(3):522-31.
28. Buysman E, Conner C, Aagren M, Bouchard J, Liu F. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin in a patients with type 2 diabetes. Curr Med Res Opin. 2011;27(9):1709-17.
29. Lee LJ, Li Q, Reynolds MW, Pawaskar MD, Corrigan SM. Comparison of utilization, cost, adherence, and hypoglycemia in patients with type 2 diabetes initiating rapid-acting insulin analog with prefilled pen versus vial/syringe. J Med Econ. 2011;14(1):75-86.
30. Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med. 2002;19(1):1-11.
|
Author for correspondence: Magaly Perez-Nieves, MPH, PhD, Research Scientist, Global Patient Outcomes and Real World Evidence, Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN 46285, USA |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2018 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.