Physicochemical stability of Cytarabine Accord in punctured original vials and after dilution with 0.9% sodium chloride solution in polyolefine bags
Published on 2024/09/11
Generics and Biosimilars Initiative Journal (GaBI Journal). 2024;13(2):105-6.
Author byline as per print journal: Irene Krämer, PhD; Frank Erdnuess, PhD; Judith Thiesen, PhD
Submitted: 4 March 2024; Revised: 15 April 2024; Accepted: 26 April 2024; Published online first: 6 May 2024
Introduction
Cytarabine, also known as arabinosylcytosine (ara-C), is an antineoplastic agent used for treatment of leukaemias and lymphomas, especially acute myeloid leukaemia, and non-Hodgkin lymphoma. Cytarabine is a pyrimidine antimetabolite that competes with cytidine for incorporation into DNA, thereby blocking the function of DNA polymerase and leading to cell cycle arrest in the S-phase. Prior to administration, Cytarabine Accord must be diluted with 0.9% sodium chloride or 5% glucose infusion solution. According to the Summary of Product Characteristics (SmPC) , Cytarabine Accord infusion solutions diluted with 0.9% sodium chloride or 5% glucose solution are physicochemically stable for 24 hours when stored at room temperature and 72 hours when refrigerated [1]. Cytarabine Accord concentrate should not be stored refrigerated [1]. According to published stability data of various cytarabine brand products, cytarabine infusion solutions ( with vehicle solutions 0.9% sodium chloride , 5% glucose) and nominal concentrations in the range of 0.2 mg/mL to 25 mg/mL are physicochemically stable for up to 30 days [2–5].
Study objectives
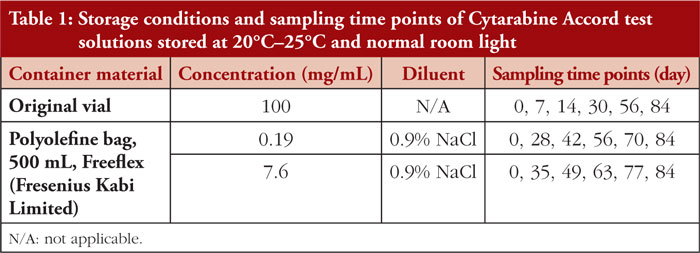
The aim of the study was to determine the physicochemical stability of Cytarabine Accord 100 mg/mL concentrate in 50 mL original vials after first opening , as well as after dilution in 500 mL polyolefine (PO) bags pre-filled with 0.9% sodium chloride solution, resulting in concentrations of 0.19 mg/mL and 7.6 mg/mL, when stored at 20°C–25°C under normal room light for a period of 84 days.
Methods
Cytarabine test solutions were prepared under EU Class A conditions and in accordance with the principles of Good Manufacturing Practice . Three different test solutions were prepared using the European Medicines Agency (EMA) licensed Cytarabine Accord 100 mg/mL (batch numbers N13936 and R08292). All test solutions were stored at 20°C– 25°C under normal room light. Samples were taken and analysed initially (Day 0) and at predetermined time points. For detailed information, see Table 1.
The analysis of physical stability comprised pH measurements ( using a glass electrode calibrated with a standard buffer solution) and visual inspections under standard laboratory light against a black and white background for any changes in colour, clarity, or the presence of particulate matter.
Chemical stability was assessed via high-performance liquid chromatography (HPLC) assay, which was validated for linearity of analytical response and acceptable precision [6]. The assay was proven to be stability-indicating for non-specific degradation of the parent drug. Acceptance criteria were set to a cytarabine concentration ±5% of the label claim for undiluted test solutions and to a cytarabine concentration ±10% of the initially measured concentration for diluted test solutions [6].
Results
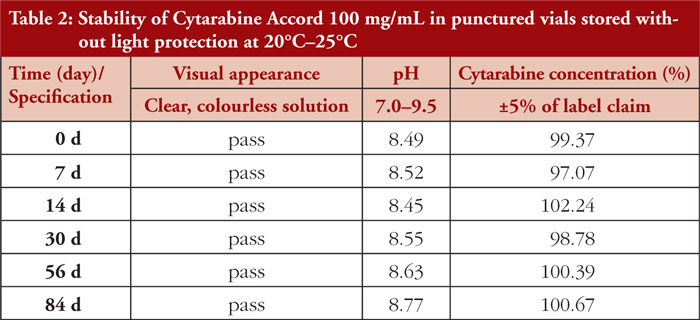
Cytarabine Accord 100 mg/mL concentrate in 50 mL punctured vials remained physicochemically stable for 84 days when stored at room temperature and under normal room light. Neither colour change, turbidity , nor visible particles were detected during visual inspection. Results of pH measurements indicated a slight increase in pH over time (8.49 to 8.77). The HPLC assays revealed only slight variations of cytarabine concentrations on different time points , which were considered to be related to assay variability, see Table 2.
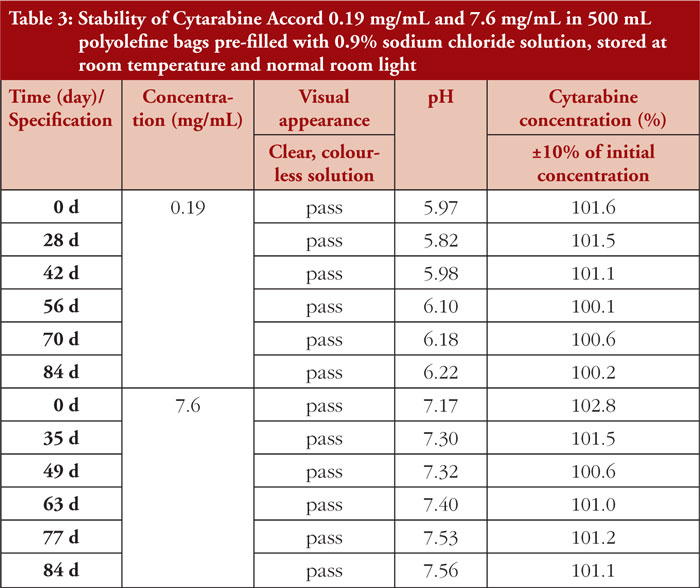
Diluted Cytarabine Accord test solutions (0.19 mg/mL and 7.6 mg/mL) in pre-filled 0.9% sodium chloride PO bags and stored at room temperature under normal room light remained physicochemically stable for up to 84 days. The results of pH measurement revealed a slight increase over time (0.19 mg/mL from pH 5.97 to pH 6.22, and 7.6 mg/mL from pH 7.17 to pH 7.56). Detailed results are provided in Table 3.
Conclusion
Cytarabine Accord 100 mg/mL, 50 mL remained physicochemically stable in the original glass vials over a period of up to 84 days after first opening when stored at room temperature and normal room light. After dilution with 0.9% sodium chloride, cytarabine solutions 0.19 mg/mL and 7.6 mg/mL remained physicochemical ly stable for 84 days when stored at 20°C–25°C under normal room light.
Residues of undiluted Cytarabine Accord 100 mg/mL can be used over a period of 84 days after first opening when stored at room temperature and normal room light. Cytarabine infusion solutions in pre-filled 0.9% sodium chloride PO bags in the concentration range of 0.19 mg/mL to 7.6 mg/mL can be prepared in advance by pharmacy-based cytotoxic units and used over a period of 84 days when stored at room temperature and normal room light.
Analysis was performed and documented by an accredited external laboratory. Results were carefully checked for plausibility and cautiously interpreted.
Funding sources
This study was funded by Accord Healthcare.
Competing interests: The authors Irene Krämer, Frank Erdnuess, and Judith Thiesen have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Professor Irene Krämer, PhD
Frank Erdnuess, PhD
Judith Thiesen, PhD
Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany
References
1. Accord Healthcare Limited. Summary of product characteristics for Cytarabine Accord 100 mg/mL concentrate for solution for infusion. Available from: https://cdn.accord-healthcare.com/ie/public/spc/downloadspc_cytarabine_100mg.pdf
2. Ayed WB, Drira C, Soussi MA, Ouesleti H, Hamdene B, Khrouf M, et al. Physical and chemical stability of cytarabine in polypropylene syringes. J Oncol Pharm Pract. 2021;27(4):827-33.
3. Rochard EB, Barthes DMC, Courtois PY. Stability of fluorouracil, cytarabine, or doxorubicin hydrochloride in ethylene vinylacetate portable infusion-pump reservoirs. Am J Hosp Pharm. 1992;49(3):619-23.
4. Dubach-Stutz K, Mühlebach S. Cytarabine stability in ready-to-use solution: differences between two competitor products. J Oncol Pharm Pract. 1995;1:35.
5. Astier L , Alarcon J, Pinguet, F, Canal P, Viaud P, Fety R, et al. Stability time of the anti-neoplastic drugs in the PVC infusion bags. Poster presentation at the Proceedings of the 19th European Symposium on Clinical Pharmacy 1990.
6. Accord Healthcare Limited. Data for HPLC assay and acceptance criteria on file; 30-05-14.
|
Author for correspondence: Judith Thiesen, PhD, Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2024 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.