Physicochemical stability of Gemcitabine Accord 100 mg/mL in punctured original vials and after dilution with 0.9% sodium chloride in polyolefine bags
Published on 2024/09/11
Generics and Biosimilars Initiative Journal (GaBI Journal). 2024;13(2):111-13.
Author byline as per print journal: Irene Krämer, PhD; Frank Erdnuess, PhD; Judith Thiesen, PhD
|
Study objectives: To determine the physicochemical stability of Gemcitabine Accord 100 mg/mL concentrate in original vials after first opening and after dilution with 0.9% sodium chloride solution in polyolefine (PO) bags at concentrations of 0.1 mg/mL and 10 mg/mL. |
Submitted: 15 April 2024; Revised: 25 April 2024; Accepted: 2 May 2024; Published online first: 13 May 2024
Introduction
Gemcitabine is a synthetic pyrimidine nucleoside antimetabolite which inhibits DNA replication and repair and induces apoptosis. Gemcitabine is indicated in the treatment of ovarian, pancreatic, breast, and non-small cell lung cancer, among others. Various gemcitabine formulations are marketed, such as powder for injection (38 mg/mL gemcitabine after reconstitution) and solution concentrates for injection (10 mg/mL, 38 mg/mL, 40 mg/mL, 100 mg/mL). Diluted gemcitabine solutions are reported to be physicochemically stable for 35 days (5% glucose vehicle solution) and 49 days (0.9% sodium chloride vehicle solution), irrespective of storage temperature [1–3]. According to the Summary of Product Characteristics (SmPC), Gemcitabine Accord 100 mg/mL diluted with 0.9% sodium chloride solution to a concentration range of 0.1 mg/mL to 9.0 mg/mL is physicochemically stable for 60 days, either stored at 2°C–8°C or at 25°C [4]. In-use stability of the solution concentrate in the original vial after first opening is not reported [4].
Study objectives
The objective of the study was to determine the physicochemical stability of Gemcitabine Accord 100 mg/mL concentrate in original vials after first opening and after dilution with 0.9% sodium chloride solution in 500 mL polyolefine (PO) bags containing 0.1 mg/mL and 10 mg/mL gemcitabine and stored under ambient light conditions at 20°C– 25°C for a maximum period of 84 days.
Methods
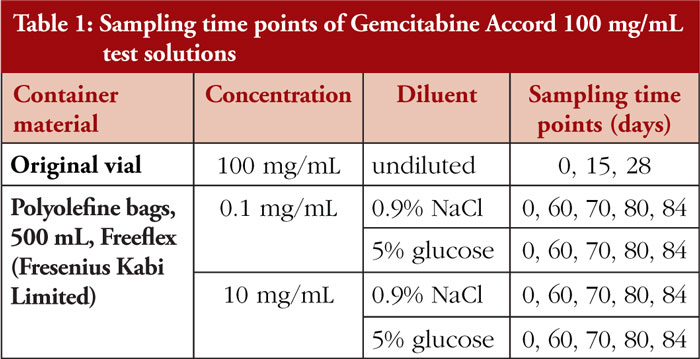
Gemcitabine test solutions were prepared under EU Class A conditions and in accordance with the principles of Good Manufacturing Practice. A total of five different test solutions were prepared using the European Medicines Agency (EMA) licensed Gemcitabine Accord 100 mg/mL (batch numbers M03386 and PR01162). These test solutions were stored at 20°C–25°C without light protection/normal room light. Samples were taken and analysed initially (Day 0) and at predetermined time points, as detailed in Table 1.
The physical stability analysis of all test solutions included visual inspections under standard laboratory light to detect any change s in colour, clarity, or the presence of particulate matter. pH measurement (using a glass electrode calibrated with standard buffer solutions) was performed on diluted infusion solutions.
Chemical stability was assessed via high-performance liquid chromatography (HPLC) analysis, which was validated for linearity of analytical response and acceptable precision. The assay was proven to be stability-indicating for non-specific degradation of the parent drug [5]. Acceptance criteria were set to a gemcitabine concentration of ±5% of the label claim for gemcitabine concentrate in original vials and ±10% of the label claim for diluted gemcitabine test solutions [5]. Samples of 100 mg/mL solutions in punctured vials were taken, and quantities of related substances analysed by HPLC assay. Peak areas of related substances were calculated as percentage rate of the gemcitabine main peak. Acceptance criteria for related substances were set as follows [5]:
- Cytosine : </= 0.1%
- Gemcitabine α-anomer : </= 0.1%
- Any other impurity : </= 0.10%
- Total impurities : </= 0.3%
Results
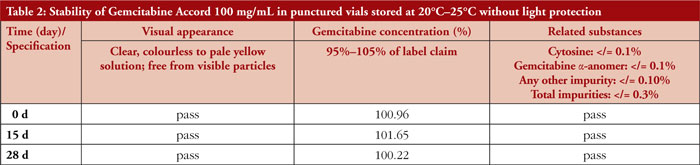
Gemcitabine Accord 100 mg/mL concentrate in punctured original vials stored without light protection at 20°C– 25°C remained physicochemically stable over the observation period of 28 days. No colour change, turbidity, or visible particles were detected upon visual inspection. The HPLC assays revealed only slight variations in gemcitabine concentrations at different time points, which were considered to be related to assay variability, further details are provided in Table 2. Several peaks of impurities were observed in the chromatograms of the solution concentrate, with peak areas far below the acceptance limits. Single peaks of other unknown impurities amounted to less than 0.03%, and the total impurities accounted for less than 0.03% of the main peak. All impurity peaks remained unchanged over time.
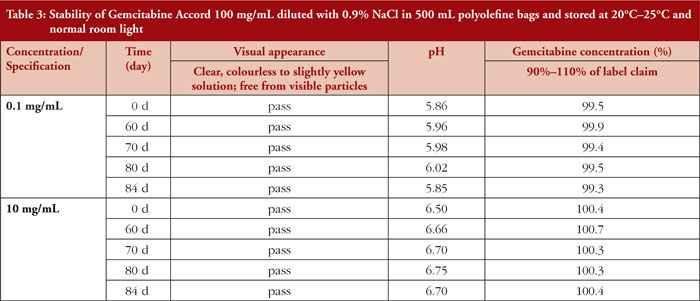
Diluted Gemcitabine Accord 100 mg/mL test solutions, stored in PO bags at room temperature and normal room light, remained physicochemically stable over the 84-day observation period, regardless of the gemcitabine concentration (0.1 mg/mL, 10 mg/mL). For detailed results, see Table 3.
Conclusion
Gemcitabine Accord 100 mg/mL concentrate stored at 20°C– 25°C was found to be physicochemically stable over the observation period of 28 days after first opening. After dilution with 0.9% sodium chloride infusion solution to the nominal concentrations 0.1 mg/mL and 10 mg/mL gemcitabine, test solutions were found to be physicochemically stable over the test period of 84 days when stored at 20°C– 25°C and normal room light.
Any unused portion of 100 mg/mL concentrate in punctured original vials can be used cost-effectively for 28 days when stored at room temperature. Diluted infusion solutions in polyolefine bags in the concentration range of 0.1 mg/mL to 10 mg/mL gemcitabine can be produced in advance in pharmacy-based cytotoxic preparation units and used over a period of 84 days when stored at room temperature and normal room light.
Analysis was performed and documented by an accredited external laboratory. Results were carefully checked for plausibility and cautiously interpreted.
Funding sources
This study was funded by Accord Healthcare.
Competing interests: The authors Irene Krämer, Frank Erdnuess, and Judith Thiesen have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Professor Irene Krämer, PhD
Frank Erdnuess, PhD
Judith Thiesen, PhD
Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany
References
1. Xu Q, Zhang Y, Trissel LA. Physical and chemical stability of gemcitabine hydrochloride solutions. J Am Pharm Assoc (Wash). 1999;39(4):509-13.
2. Closset M, Colsoul ML, Goderniaux N, Bihin B, Jamart J, Onorati S, et al. An ultra-high-performance chromatography method to study the long term stability of gemcitabine in dose banding conditions. J Pharm Biomed Anal. 2023;227:115290.
3. Pontón JL, Munoz C, Rey M, Val M. The stability of (lyophilized) gemcitabine in 0.9% sodium chloride injection. Eur J Hosp Pharm. 2002;1:23-25.
4. Accord Healthcare Limited. Summary of product characteristics for Gemcitabine Accord 100 mg/mL concentrate for solution for infusion. Available from: https://www.accord-healthcare-products.co.uk/products/g/gemcitabine-concentrate
5. Accord Healthcare Limited/Astron Research Limited. Data for HPLC assay and acceptance criteria on file; 30-05-14 and 20-08-14.
|
Author for correspondence: Judith Thiesen, PhD, Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2024 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.