Physicochemical stability of Idarubicin Accord in punctured original vials, syringes and after dilution with water for injection
Published on 2024/09/11
Generics and Biosimilars Initiative Journal (GaBI Journal). 2024;13(2):68-70.
Author byline as per print journal: Irene Krämer, PhD; Frank Erdnuess, PhD; Judith Thiesen, PhD
|
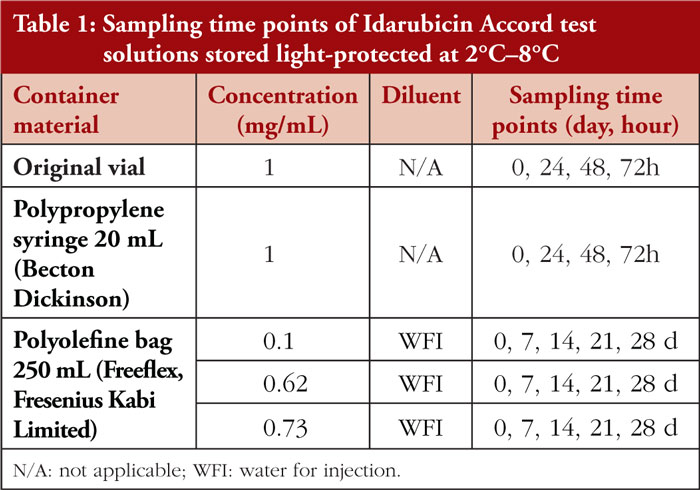
Study objectives: To determine the physicochemical stability of undiluted Idarubicin Accord in punctured original vials and polypropylene syringes as well as after dilution with water for injection (WFI) in polyolefine (PO) bags at three different concentrations (0.1 mg/mL, 0.62 mg/mL, and 0.73 mg/mL) and storage at 2°C–8°C for up to 28 days. |
Submitted: 12 February 2024; Revised: 18 April 2024; Accepted: 26 April 2024; Published online first: 6 May 2024
Introduction
Idarubicin is an anthracycline antineoplastic agent inhibiting DNA synthesis by intercalation and interfering with the topoisomerase II enzyme. Main indications are acute leukaemias (AML, ANLL), where it is mostly used in combination with cytarabine [1]. Moreover, idarubicin is used in transarterial chemoembolization (TACE) of hepatocellular carcinoma [2].
Idarubicin consists of the water-insoluble aglycone (4-demethoxydaunorubicinone) and a water-soluble amino sugar (daunosamine). In acidic solutions, the glycosidic bond is hydrolised. In alkaline solutions, the aglycone portion of the molecule is degraded. Diluted idarubicin solutions (0.1 mg/mL in 5% glucose or 0.9% NaCl as vehicle solutions) are known to be physicochemically stable for 28 days when stored light protected [3]. Higher-concentrated idarubicin solutions (4 mg/mL) have been shown to be chemically stable for at least 7 days when stored refrigerated in polypropylene (PP) syringes [4].
Study objectives
To determine the physicochemical stability of undiluted Idarubicin Accord 1 mg/mL injection concentrate in punctured original vials and PP syringes, as well as after dilution with water for injection (WFI) in polyolefine (PO) bags at three different concentrations, and storage at 2°C–8°C for a maximum period of 28 days.
Methods
Idarubicin HCL test solutions were prepared under EU Class A conditions and in accordance with the principles of Good Manufacturing Practice. A total of five different test solutions were prepared using Idarubicin Accord 1 mg/mL (batch number PN00936). The test solutions were protected from light and refrigerated (2°C–8°C) for up to 28 days. Samples were taken and analysed initially (Day 0) and at predetermined time points. For detailed information, refer to Table 1.
The physical stability analysis comprised pH measurements (using a glass electrode calibrated with standard buffer solutions) and visual inspections under standard laboratory light against a black and white background to detect any changes in colour, clarity, and the presence of particulate matter.
Chemical stability was assessed via high-performance liquid chromatography (HPLC) assay, which has been validated for linearity of analytical response and acceptable precision. The assay was proven to be stability-indicating for non-specific degradation of the parent drug [5]. The acceptance criteria for all test solutions were defined as initial ±10% of the label claim [5].
Results
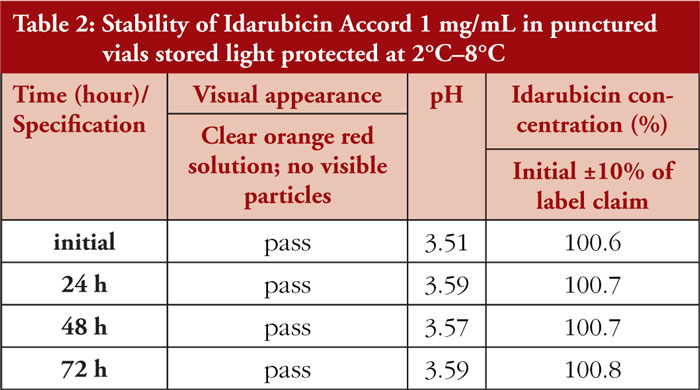
Idarubicin Accord 1 mg/mL concentrate in punctured vials remained unchanged when stored refrigerated and light protected over the test period of three days. Neither a colour change nor turbidity or visible particles were noticed during visual inspection. Results of pH measurements showed only slight variations (less than 0.1 unit) during the observation period. In the HPLC chromatograms, no signs of chemical degradation of idarubicin were found. Slight variations of idarubicin concentrations at different time points are related to the assay variability, as shown in Table 2.
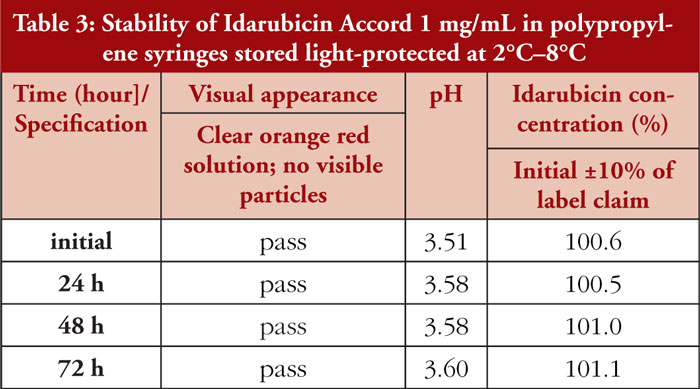
Undiluted Idarubicin Accord 1 mg/mL is stored refrigerated and light protected in 20 mL PP syringes. It remained unchanged over the observation period (three days). Detailed results are given in Table 3.
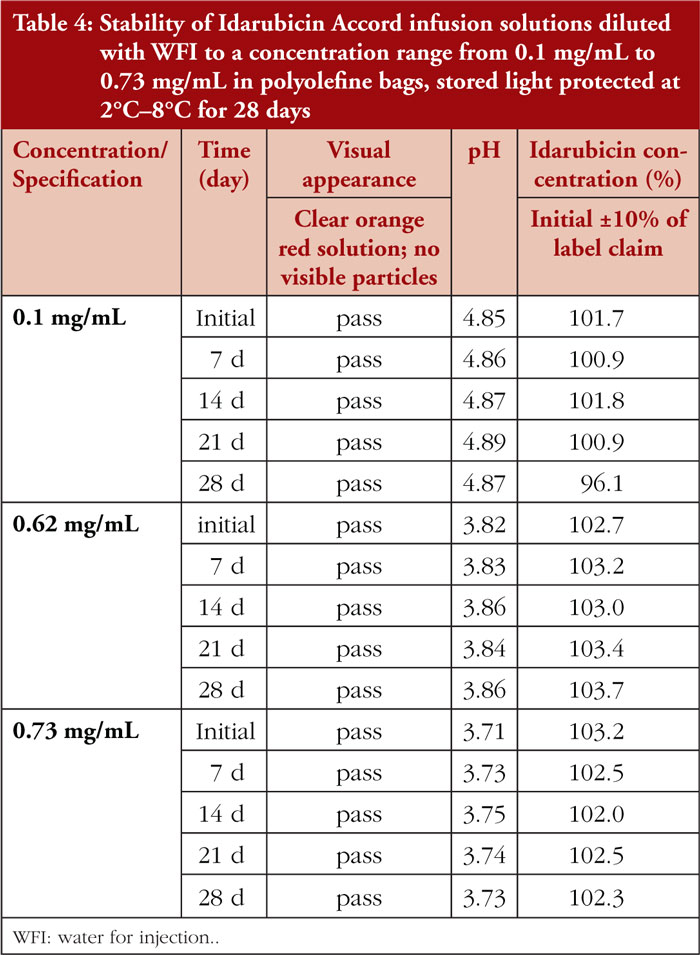
Diluted idarubicin test solutions remained physicochemically stable for 28 days when protected from light in PO bags at 2°C–8°C. For detailed results, see Table 4.
Conclusion
Idarubicin HCl Accord solution concentrate 1 mg/mL was found to be physicochemically stable for at least three days after first opening. No signs of impaired stability were found when stored refrigerated and light protected, regardless of the storage container used (i.e. punctured original vials and 20 mL PP syringes).
After dilution with WFI to concentrations of 0.1 mg/mL, 0.62 mg/mL, and 0.73 mg/mL, extended stability was proven. When stored light protected in PO bags at 2°C–8°C, all test solutions remained physically and chemically stable for 28 days. These findings are in line with previously published stability data [2]. Moreover, physicochemical stability of idarubicin solution is demonstrated for higher concentrations (up to 0.73 mg/mL) than previously published (0.1 mg/mL only) [2].
Residues in the punctured original vials can be used cost-effectively for up to three days when stored refrigerated. Diluted infusion solutions with idarubicin concentrations between 0.1 mg/mL and 0.73 mg/mL may be prepared in advance by pharmacy-based cytotoxic preparation units and used over a period of 28 days if refrigerated storage in PO bags is provided. Test solutions were prepared with water for injection as vehicle solution. As osmolality was not measured in this study, highly diluted idarubicin infusion solutions (0.1 mg/mL) with WFI as the vehicle solution should be used carefully. Storage of solutions at room temperature has not been tested and thus should be avoided. Further investigations on extended physicochemical stability at room temperature are needed, as in clinical practice interruption of the cold chain during transportation and storage cannot always be avoided.
Analysis was performed and documented by an accredited external laboratory. Results were carefully checked for plausibility and cautiously interpreted.
Funding sources
This study was funded by Accord Healthcare.
Competing interests: The authors Irene Krämer, Frank Erdnuess, and Judith Thiesen have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Professor Irene Krämer, PhD
Frank Erdnuess, PhD
Judith Thiesen, PhD
Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany
References
1. Accord Healthcare Limited. Summary of product characteristics for 5 mg/5 mL solution for injection. Available from: https://accord-healthcare-products.co.uk/products/i/idarubicin-hydrochloride-solution
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018;391(10127):1301-4.
3. Beijnen JH, Rosing H, De Vries P, Underberg WJ. Stability of anthracycline antitumour agents in infusion fluids. J Parenter Sci Technol. 1985;39(6):220-2.
4. Bourcier B, Lagarce F, Lebreton V. Stability of a high-concentrated aqueous solution of idarubicin stored in a polypropylene syringe for transarterial chemoembolization. J Pharm Biomed Anal. 2022; 210:114543.
5. Accord Healthcare Limited. Data for HPLC assay and acceptance criteria on file; 09-06-2015.
|
Author for correspondence: Judith Thiesen, PhD, Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2024 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.