Regional management of biosimilars in Germany
Published on 2016/10/03
Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(3):125-7.
|
Abstract: |
Submitted: 10 August 2016; Revised: 27 September 2016; Accepted: 30 September 2016; Published online first: 14 October 2016
Biological pharmaceuticals have become increasingly important in medical treatment over the past 15 years [1]. They have opened up relevant, new treatment options in many areas, including oncology and nephrology, and also in anti-inflammatory treatment.
Anti-inflammatory biologicals are commonly offered as original preparations with annual treatment costs of more than Euros 20,000 per patient in Germany [2]. As a means of managing this financial burden on the healthcare system, and to finance new medications, it is important to have alternative treatment options available following patent expiry, and to use these actively. This paper describes how this process has occurred to facilitate the use of biosimilars, and also presents the current prescribing governance of tumour necrosis factor-alpha (TNF-α) blockers.
Biosimilars have a comparable pharmacologic effect to their respective reference products. However, neither biosimilars nor sequential batches of the same reference product exhibit a 100% identical structure. A highly differentiated approval process has therefore been established for biosimilars in Europe, through the European Medicines Agency (EMA), which ensures the quality, efficacy and also the comparability of biosimilars with their reference products.
This paper focuses on regional experiences in Westphalia-Lippe, part of the German Federal State North Rhine-Westphalia. Currently, this region has seven million patients with statutory health insurance (SHI). The number of people in Germany with SHI is 70 million and this region represents approximately 11% of the German population [3].
At a regional level, the first approved biosimilars, such as epoetins, were actively managed. The response to these new products in this case study region was positive, and a significant proportion of prescriptions were switched to biosimilars without any problems being reported. The proportion of biosimilar prescriptions is, on average, over 60% today [4]. Of particular importance to governance are biosimilars of the TNF-α inhibitors, infliximab and etanercept, which have been available since 2015 and 2016, respectively. Regionally, the prescribing cost for infliximab in 2015 was almost Euros 30 million in Westphalia-Lippe. This presents an important saving potential, as the sum is only 1% of the regional overall pharmaceutical budget. Regional prescription management attempts to take advantage of potential savings, especially if they are achievable without changing treatment paradigm or quality. As such, biosimilars represent a simple and proven opportunity for savings here.
Regional experiences with TNF-α biosimilars
At a regional level, we have long been working with quotas for biosimilars, such as epoetins, somatropins, introduced since 2006. Historically, this has worked well with biosimilar epoetins prescribed by nephrologists or oncologists. Currently, TNF-α biosimilars are also primarily managed with quotas in this case study region. However, challenges occurred with physicians, who include gastroenterologists, dermatologists and rheumatologists, as they had no experience of prescribing biosimilar infliximab.
Assessment of new biosimilar management in this region today is therefore limited by comparison to the experience of the management of biosimilars that have been available for a longer time period. Here, in the case study region, we have established different methods of actively informing doctors and supporting market entry by providing extensive information to physicians and conducting regular reporting, it has been possible to gain a high acceptance for the prescribing of biosimilars. From our perspective, this supports the hypothesis that doctors have a high level of trust in the decisions made by approval authorities like EMA. Issues, such as the extrapolation of indications, were practically irrelevant at a regional level. On the other hand, there was a high level of sensitivity among doctors to the available information on savings potentials in relation to reference products.
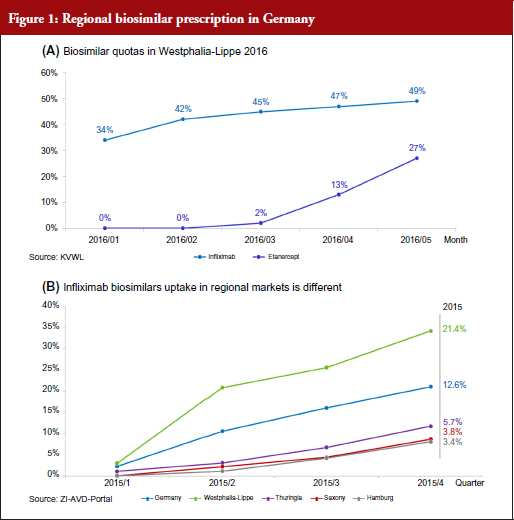
Figure 1 displays the fast uptake of infliximab biosimilars prescriptions, but also marks the significant regional differences.
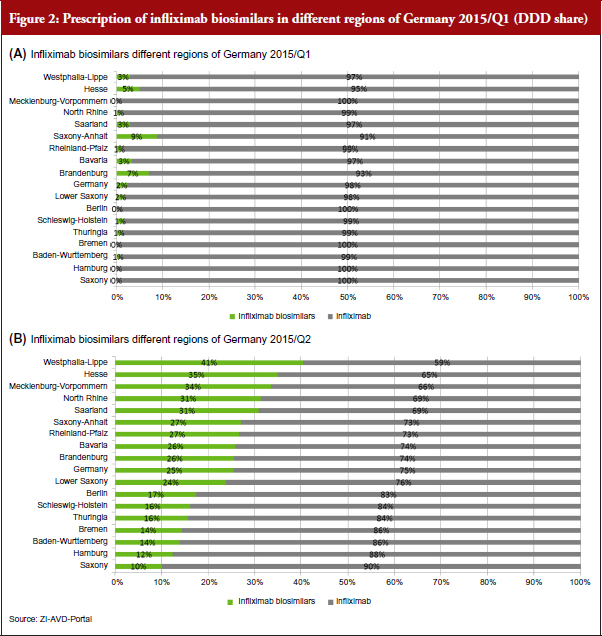
Nationwide observations, see Figure 2, show relevant differences in the prescription patterns. These are taken from ZI, an official scientific institute of the German physicians associations which also analyses prescription data from public pharmacies for SHI patients [5, 6]. It is an official and accepted source of information in Germany. We believe that active management and transparent information, inthe case study region, arethe main reasons for high biosimilar uptake. Other regulations in place to manage prescriptions are similar in all the federal countries of Germany, which is thought to be a cause of lower relative uptake.
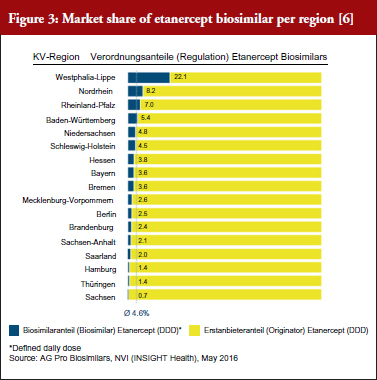
Initial experience of managing the biosimilar etanercept indicates that a solid foundation has previously been laid by providing broader information and allowing for successful management of biosimilars in general. As a result, etanercept biosimilars are prescribed significantly more often in the case study region, see Figure 3.
Conclusion
The prescription uptake of TNF-α biosimilars shows regional differences. Experiences in Westphalia-Lippe indicate that practical information and management of biosimilars can enable a high level of acceptance and prompt switching to biosimilars in practice. Initial data on etanercept seem to reflect uptake levels of infliximab that was introduced successfully at an earlier date.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
1. Schwabe U, Paffrath D. Arzneiverordnungs-Report 2015. Springer-Verlag Berlin Heidelberg; 2015. p. 24-5.
2. Schwabe U, Paffrath D. Arzneiverordnungs-Report 2015. Springer-Verlag Berlin Heidelberg; 2015. p. 513.
3. GKV-Spitzenverband [homepage on the Internet]. [cited 2016 Sep 27]. Available from: https://www.gkv-spitzenverband.de/presse/zahlen_und_grafiken/zahlen_und_grafiken.jsp#lightbox
4. GKV-Spitzenverband. Rahmenverträge zur Arzneimittelversorgung. Rahmenvorgaben nach § 84 Abs. 7 SGB V. Arzneimittel – für das Jahr 2017 vom 30 September 2016. Page 11 [homepage on the Internet]. [cited 2016 Sep 27]. Available from: https://www.gkv-spitzenverband.de/krankenversicherung/arzneimittel/rahmenvertraege/rahmenvertraege.jsp
5. ZI – Zentralinstitut für die kassenärztliche Versorgung [homepage on the Internet]. [cited 2016 Sep 27]. Available from: http://www.zi-berlin.de/cms/index.php
6. AG Pro Biosimilars. Grafik des Monats Juli 2016. [cited 2016 Sep 27]. Available from: http://proBiosimilars.de/presse/grafik-des-monats-juli-2016/
|
Author: Mathias Flume, MBA, PhD, Kassenärztliche Vereinigung Westphalia-Lippe, DE-44127 Dortmund, Germany |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2016 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.