Regulation of biologicals in Malaysia
Published on 2014/09/23
Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(4):193-8.
|
Abstract: |
Submitted: 19 September 2014; Revised: 23 October 2014; Accepted: 28 October 2014; Published online first: 10 November 2014
Background
In Malaysia, the evaluation of biologicals has been incorporated into a regulatory system established for the purpose of providing marketing authorization for medicinal products. The past decade has witnessed not only a market dominance of biologicals, led by a wave of biologicals discovered in the 80s and 90s to address the treatment of diseases with unmet needs, but also an increase in the market penetration of ‘copy products’ of blockbuster biologicals with expired patent protection. The use of recombinant enzymes as replacement therapy for enzyme deficiency diseases such as Fabry disease and Pompe disease addresses the core physiological deficiency and offers specific disease treatment, a feat not possible to achieve with current, chemically synthesized compounds. However, the rapid development of biological compounds and their follow-on versions have posed considerable challenges to drug regulators worldwide.
As a result, a dedicated regulatory pathway has been created for biologicals and biosimilars, which is really a paradigm shift from evaluating each product on its own merit based on its biological nature – inherently variable, complex, and unique. Even then, regulators constantly face the pressure of keeping up with trends and advances in biotechnological manufacturing and analytical tools.
Introduction to the organization
The National Pharmaceutical Control Bureau (NPCB) is the National Regulatory Authority (NRA) in Malaysia. NPCB was established as a secretariat to the Drug Control Authority (DCA), an executive body chaired by the Director-General of Health, for the approval of all pharmaceutical, health and personal care products, as described in the Control of Drugs and Cosmetics Regulation of 1984 [1].
NPCB is responsible for ensuring the quality, efficacy and safety of medicinal products prior to release in the Malaysian market. In upholding the three tenets of quality, efficacy and safety, NPCB regularly engages with both internal and external partners. Internal partners include public health and health technology assessment agencies within the Ministry of Health Malaysia, while external partners/counterparts include healthcare professionals, academia, industry representatives, the World Health Organization (WHO) and other NRAs. The mission of NPCB is to implement effective regulatory controls that contribute to improving health care for Malaysians.
Among its regulatory control strengthening strategies, NPCB strives to improve the efficiency of work processes via its Quality Management System (QMS). NPCB currently maintains the standards of MS ISO 9001:2008. As not all the agencies within the Ministry of Health are MS ISO 9001:2008-compliant, this makes NPCB one of the few health agencies with a QMS that matches international expectations.
NPCB is a WHO Collaborating Centre for Regulatory Control of Pharmaceuticals. Through the Association of Southeast Asian Nations (ASEAN) Technical Cooperation among Developing Countries (TCDC) programme, NPCB has served as a regional training centre since 1986. Several NPCB officers have travelled overseas as consultants to assist in the development of pharmaceutical regulatory control systems.
Evaluation of biologicals in Malaysia
The classification of a medicinal product as a biological is clearly defined in the Drug Registration Guidance Document (DRGD), together with a list of included and excluded product types [2]. Appendix 3 of this document includes regulatory guidance on the registration of biologicals. For the purpose of product registration, the terms ‘biotechnology’ and ‘biologicals’ can be used interchangeably, as evaluation of both product categories are conducted by the same team of evaluators. In the context of product registration, biologicals encompass both naturally occurring substances extracted from living organisms and biotechnology-derived products. This includes biotechnology products, blood-derived products and vaccines. Currently, the following product types are not evaluated by the Biotechnology Section: (1) metabolites from microorganisms, e.g. antibiotics and some hormones; (2) macromolecules produced by chemical synthesis, e.g. peptides such as lung surfactants and oligonucleotides; and (3) whole blood or cellular blood components.
At NPCB, biological evaluation activities are performed by the Biotechnology Section, a division within its Centre for Product Registration. As biologicals include a wide range of product categories, the sub-divisions of the Section reflect its current product portfolio: vaccines, blood-derived products, and biotechnology products including biosimilars. The product types currently registered at NPCB include: monoclonal antibodies (mAbs), recombinant human insulin and insulin analogues, hormones, cytokines, antibody-drug conjugates, vaccines and plasma fractionation/recombinant analogue products. Figure 1 shows the breakdown and total number of biological products registered in Malaysia in the past five years. For a list of drugs registered in any particular year, readers may refer to the annual reports published on the NPCB website (http://portal.bpfk.gov.my/index.cfm).
The numbers in brackets at the top of each column indicate the total number of registered products in that year. Products containing different strengths of the same drug substance are considered as one product (see keys in Figure 1).
Quality assurance
The general principles of evaluation of biologicals at NPCB are consistent with current international regulatory practices. As a requirement for quality assurance, every biological must comply with the principles of Good Manufacturing Practice (GMP) for biologicals as established in the guidance documents of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (jointly referred to as PIC/S) [2]. NPCB has been a PIC/S member authority since 1 January 2002 and every biological product submitted for evaluation must possess a valid GMP certificate as issued by a PIC/S member authority. For products submitted without prior PIC/S GMP certification, the GMP inspection team from NPCB will inspect and certify the manufacturing facility. This is a prerequisite for product dossier submission. NPCB believes that a PIC/S GMP certification is key to risk reduction and establishment of a quality system that ensures consistent production. In addition to quality assurance by GMP, all steps involved in the gathering of the starting materials, e.g. plasma from screened donors, product manufacturing, and excipient source are scrutinised to the strictest level in line with international standards in order to further ensure non-transmission of adventitious agents to users.
Registration process and requirements
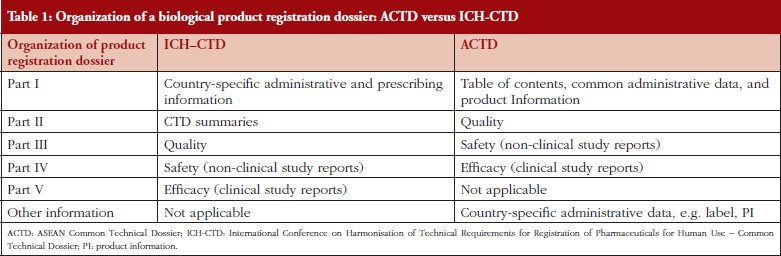
Within the ASEAN economic community, a biological product submission must adhere to the organization and document requirements as outlined in the ASEAN Common Technical Dossier (ACTD) format [3]. Table 1 highlights the differences in format between the ACTD and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Common Technical Dossier (ICH-CTD), as formulated by regulators from the ICH (Europe, Japan and the US) regions [4].
The table lists the parts of a biological product submission dossier for the purpose of registration. The dossier requirement according to ACTD is compared with the dossier requirement according to ICH-CTD.
The inherent complexity of biological molecules calls for regular creation of new and product-focused regulatory guidance documents to account for evaluation of new molecules and technologies. Regulatory guidance has always evolved with advances in recombinant technology and bioprocesses. In addition to the ACTD dossier and DRGD for product registration in Malaysia, requirements for registration of biologicals are consistent with the guidances established by WHO and ICH. Other regulatory expectations such as those published by mature and referenced regulatory agencies, for instance, the European Medicines Agency (EMA) and the US Food and Drug Administration (US FDA), can also be referred to. NPCB strives to emulate the examples set by more established regulatory authorities in order to avoid replication, preferring to adopt and adapt the regulatory guidance from these agencies as appropriate for local use. At the same time, NPCB has been represented in the formulation and implementation of regulatory guidance documents at WHO level, via participation in implementation workshops, especially for biosimilars.
Evaluation of every biological product is performed via the established procedure for a pharmaceutical product registration at NPCB. Submission of a biological product registration dossier is conducted through an online and manual system. Since 2011, NPCB has been operating an online product registration system under the acronym QuEST3 (Quality, Efficacy and SafeTy 3; the digit ‘3’ indicates the current system version). The paper-based manual submission system is still retained and complements the online QuEST3 submission system.
The registration process for a biological at NPCB begins when an online submission by an applicant is received. Once the dossier is deemed to fulfill the requirements for registration, a manual submission process takes place, where the applicant submits the printed documents for evaluation. The applicant is also required to submit the analytical methods and validation documents for evaluation at the Centre for Quality Control, NPCB. NPCB regularly consults external experts (academicians, clinical consultants, and public health and blood product experts) throughout its evaluation process. External experts and key opinion leaders (KOLs) are selected for independence, with the highest level of confidentiality in place. Internal product evaluation meetings are held among the various centres at NPCB to formulate an evaluation report that can be presented to DCA for consideration. The possible outcomes at the DCA level are: (1) product is registered; (2) product is rejected, with reasons or; (3) product is withheld pending further information from applicant. An approved biological product will be assigned a unique registration number beginning with the prefix ‘MAL’ and ending with the letter ‘A’ for poison or ‘X’ for non-poison classification in accordance with the Sale of Drugs Act 1952 [5]. Parties objecting to a DCA decision may appeal to the Minister of Health within 14 days of the DCA decision. Figure 2 summarizes the biological product registration process at NPCB.
The flowchart shows the biological product registration process in Malaysia. The evaluation process encompasses internal deliberation on a product’s quality, efficacy, and safety characteristics, and consultation with external subject matter experts. Correspondence (via letter, email or face-to-face meeting) is maintained with the applicant several times during the product evaluation process in order to obtain additional product information as necessary. Information on membership and terms of reference of DCA is available on the NPCB website (http://portal.bpfk.gov.my/index.cfm).
NPCB shares a common challenge with other NRAs, i.e. to ensure satisfactory quality, safety and efficacy in approving a drug product within certain time limits. For a biological product, the timeline has been set at 245 working days. The timeline is shortened to as little as 90 working days if a certain product has been assigned a ‘priority review’ status. In the past, some biological products were assigned such status due to great promise in fulfilling an unmet medical need, e.g. a rare disease/oncology indication, or to address a threat to public health.
Besides new product registration, the Biotechnology Section also handles evaluations for an extension or update to an indication and dosage of an approved product. Generally, the processing of such an application follows the new product registration pathway, see Figure 2. In addition, we evaluate changes to a product’s profile throughout its life cycle (as long as a product remains registered). Changes to product quality, including manufacturing process improvement, updates to a product’s label and prescribing information, and extension of product dosage forms and packaging must also undergo evaluation via the variation pathway.
Recent developments on regulation of biologicals in Malaysia
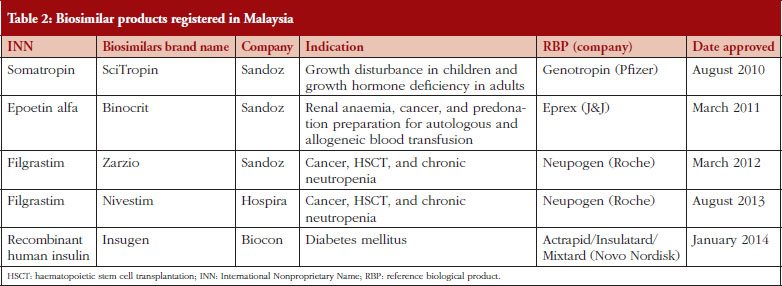
The Section on Biotechnology Product Registration at NPCB has received applications to register biosimilar products since the establishment of its biosimilar regulatory pathway in 2008. As one of the first countries to define a regulatory process for biosimilars, Malaysia has avoided marketing of any ‘biogenerics’ or ‘me-too biologicals’ with untested quality, safety, and efficacy profiles compared to those of innovator biologicals. The Malaysian guidance document and guidelines for registration of biosimilars (2008) adopted the regulatory expectations at EMA, with incorporation of local expectations. It contains a provision to align requirements with the global similar biotherapeutic product (SBP) guideline published later (in 2009) by WHO. A comprehensive discourse on this topic is discussed elsewhere [6]. To update, a total of five biosimilar products have been registered by DCA as at 31 July 2014. The breakdown on the product types is shown in Table 2. Only biosimilar compounds of relatively smaller size and lesser complexity have been registered. The reference products for these biosimilars have been marketed in Malaysia for several years with an appropriate volume of use. The extrapolation of untested indications for these biosimilars to some or all of the approved indications for the reference products was allowed during product approval. Considerations are specific to each case, based on evidence and justifications provided by the applicant. For example, Binocrit was approved for all indications for the reference product Eprex except subcutaneous use in renal disease. Based on biopharmaceutical market trends and projections, and the availability of many innovator monoclonal antibody products in the Malaysian market, we anticipate applications for biosimilar monoclonal antibodies to follow suit.
For each product, the brand name in the local market and marketing authorization holder in Malaysia is provided. The indication refers to the diseases for which treatment with the biosimilar product has been approved. The name of the original product developer is listed under the column labelled RBP (reference biological product).
International relations
NPCB is working towards increasing its international presence by frequent exchanges with regulatory counterparts worldwide. The International Conference of Drug Regulatory Authorities (ICDRA), which has been held by WHO since 1980, provides both newer NRAs, such as NPCB, as well as more mature NRAs, such as EMA, US FDA, and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) with a way to interact and explore strategies for strengthening collaboration. In the recent 16th ICDRA, Rio de Janeiro, Brazil, 24–29 August 2014, NPCB participated in the pre-conference programme on biosimilars.
Besides the activities led by WHO, other regulatory harmonization activities are conducted and frequently participated in by NPCB regulators at the Asia-Pacific and ASEAN regional levels. Within the Asia-Pacific Economic Cooperation (APEC) region, the APEC Harmonization Center (AHC) frequently coordinates regulator-industry participated forums in an effort to streamline regulatory requirements and drug product registration processes among member economies. Our regulators have participated in the AHC Biotherapeutics Workshop series and found the presentation and discussion sessions of immense benefit for application to our daily activities at NPCB.
Within the ASEAN region, Malaysia is the lead country for the ASEAN Consultative Committee for Standards and Quality – Pharmaceutical Product Working Group (ACCSQ-PPWG), with efforts towards harmonization of regulations across member states. Ongoing efforts in the regulation on biologicals focus on collating and harmonizing regulatory guidance in vaccine regulation. The group aims to establish a common set of vaccine-related registration guidelines for use within the ASEAN economic zone. In these documents, a vaccine developer can find specific requirements on the organization of a vaccine submission dossier in addition to specific stability testing requirements in ICH climatic Zone IV, i.e. hot and humid countries.
As our country is a member of the Organization of the Islamic Conference (OIC), NPCB has been represented in meetings concerning harmonization of standards on pharmaceuticals including vaccine regulation. This initiative aims to encourage self-sufficiency on pharmaceutical and vaccine products among member countries through increases in production.
Internal and external relations
In exercising its function as a pre-marketing authorization evaluation section, the Biotechnology Product Registration Section regularly engages in communication with its internal and external counterparts.
Within the organization, the Centre for Inspection and Licensing conducts inspections on biological manufacturers, from drug substance to drug product manufacturing and packaging activities. This is important to uphold the expected quality standards as established and maintained by the PIC/S. In the spirit of harmonization of GMP inspection standards, a mutual recognition system of inspections conducted among PIC/S member authorities allows NPCB to accept GMP inspection results from member PIC/S NRAs. NPCB’s GMP inspection team currently travels to manufacturing facilities across Malaysia and also to countries with non-PIC/S member NRAs to inspect biological manufacturers. These foreign inspections are conducted as a prerequisite for submission of a biological product application.
In our evaluation of quality control methods used for a biological product, our work is complemented by the evaluation activities at the Centre for Quality Control. At the centre, evaluators specializing in various analytical methods assess manufacturer’s quality control reports on analytical procedures and analytical method validation. The long-term goal here is to eventually perform market sampling and testing on registered biological products, a process which is currently lacking. This is currently a tall order, mainly due to the lack of a dedicated biological testing facility and funding constraints, but the centre has identified some products with simpler biological structure, e.g. insulin and erythropoietin, as a midterm strategy to kick start laboratory testing activities. Nevertheless, the evaluation of analytical procedures and analytical method validation still assists decision-making at the pre-marketing authorization level.
As for safety and efficacy evaluation of the products, we engage our local health experts in providing inputs on both aspects. For this purpose, NPCB frequently identifies and maintains a list of KOLs across medical fields, some of whom are actively involved in clinical trials on pre-marketed biologicals conducted in health institutions across Malaysia. DCA considers the health experts’ comments, but the decision to grant a marketing approval or not is strictly that of DCA.
In engaging our stakeholders, regular dialogues serve as a platform for new information sharing and promulgation of regulatory updates. The attendance in these dialogues includes manufacturers, industry associations and consumer representatives. Our experience demonstrates that transparent and open dialogues with all relevant stakeholders are key to putting in place a robust and pragmatic regulatory framework. At other times, advice on product registration and safety/efficacy can be obtained from the NPCB regulators through various communication channels, e.g. telephone, email, or face-to-face meetings. NPCB runs telephone call centres to respond to enquiries on product registration and product safety, including those from the general public. Information on ways to contact NPCB regulators is made available on the organization website (http://portal.bpfk.gov.my/index.cfm).
NPCB is actively seeking bilateral governmental partnerships as a forum for establishing a closer working relationship for the mutual strengthening of regulatory activities. Memorandum of Understandings (MoUs) have been signed between NPCB and the Health Sciences Authority (HSA) Singapore, as well as between NPCB and the Ministry of Health Brunei Darussalam. Following establishment of these MoUs, several regulatory exchange sessions in the form of bilateral meetings have been successfully held and the meetings explore, among other things, collaboration in the form of training programmes for regulators in these agencies. With regards to training of NPCB regulators, an annual training programme has been set up to fund NPCB regulators for overseas attachment at NRAs in Australia, the European Union, Japan, Switzerland, Taiwan and Thailand. The knowledge gained through these attachments has been disseminated at the internal level in our effort to increase our regulatory capacity for evaluation and control of a diverse biological product portfolio.
Challenges ahead
In keeping up with new biologicals and cutting-edge manufacturing methods, the regulators at NPCB participate in meetings held by WHO and other health-focused initiatives. At WHO level, NPCB regularly consults experts and receives advice and guidance on evaluation of new product types, e.g. biosimilars of different product types. NPCB regulators also participate in discussions held by WHO partner initiatives such as the Dengue Vaccine Initiative (DVI) meetings among Early Adopter Countries (EACs) in preparation for receiving the first novel dengue vaccine registration dossier.
In view of the challenges ahead, much needs to be achieved within our organization. As a basis for capacity building, a training programme for evaluators is maintained, with continuous updates based on projection of biological product registration. The focus of current training includes evaluation of a Quality by Design (QbD) product dossier, regulatory considerations on specific biosimilar products, e.g. monoclonal antibodies, and technical knowledge on cellular and gene therapy product (CGTP) manufacturing processes. A positive development is that biological regulation has been assigned as one of the priority-need areas by our management. Scholarships for postgraduate study programmes are offered annually to NPCB staff for specialized training in biologicals evaluation at local and overseas universities. The management at NPCB believes in staff motivation via sufficient appreciation and attractive remuneration. In the pharmacy services programme within the Ministry of Health, an evaluator is enrolled with a clear career pathway towards attaining the highest level of work satisfaction. In all, a conductive working environment for staff is necessary to instill a professional yet caring attitude whilst discharging their duties towards improving the health of the Malaysian population.
In conclusion, recent developments in biotechnology and health care have opened up a new and exciting vista to regulators. There is an urgent need to match appropriate regulatory control with biotechnological innovations to ensure adequate quality, efficacy, and safety of novel biologicals.
As resources for capacity building are not always available within our organization, partnerships in the form of mutual recognition agreements, experience-sharing sessions, and harmonization of regulatory requirements are actively promoted with our external stakeholders to jointly face the challenges ahead.
|
Additional information |
Acknowledgements
The authors wish to thank their past and present colleagues and collaborators, for valuable discussion and sharing sessions that have contributed in one way or another to the writing of this article.
Prior presentation: Part of this paper was presented at the 2013 APEC Harmonization Center Biotherapeutics Workshop, Seoul, Korea, on 26 September 2013 by Dr Yvonne Siew Khoon Khoo.
Funders: None.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
Authors
Arpah Abas, BPharm
Head of Biotechnology Section
Yvonne Siew Khoon Khoo, BPharm (Hons),
MMedSc, PhD (Pharmacology)
National Pharmaceutical Control Bureau
Ministry of Health Malaysia
Lot 36 Jalan Universiti, 46200 Petaling Jaya, Selangor, Malaysia
References
1. Legislation Malaysia. Control of Drugs and Cosmetics Regulation. Malaysia: Attorney General’s Chambers; 1984 [homepage on the Internet]. [cited 2014 Nov 29]. Available from: http://www.lexadin.nl/wlg/legis/nofr/oeur/lxwemal.htm
2. National Pharmaceutical Control Bureau. Ministry of Health Malaysia. Drug Registration Guidance Document (DRGD) [homepage on the Internet]. [cited 2014 Aug 24]. Available from: http://portal.bpfk.gov.my/index.cfm?&menuid=137
3. Health Sciences Authority. ASEAN: The ASEAN Common Technical Dossier (ACTD) for the Registration of Pharmaceuticals for Human Use [homepage on the Internet]. [cited 2014 Aug 24]. Available from: http://www.hsa.gov.sg/
4. ICH. ICH: The Common Technical Document for the Registration of Pharmaceuticals for Human Use. 2002/3 [homepage on the Internet]. [cited 2014 Aug 24]; Available from: http://ich.org/
5. Legislation Malaysia. Sale of Drugs Act. Malaysia: Attorney General’s Chambers; 1952 [homepage on the Internet]. [cited 2014 Nov 29]. Available from: http://portal.bpfk.gov.my/index.cfm?&menuid=137
6. Abas A. Regulatory guidelines for biosimilars in Malaysia. Biologicals. 2011;39(5):339-42.
|
Author for correspondence: Yvonne Siew Khoon Khoo, BPharm (Hons), MMedSc, PhD (Pharmacology); Biotechnology Section, Centre for Product Registration, National Pharmaceutical Control Bureau, Ministry of Health Malaysia, Lot 36 Jalan Universiti, 46200 Petaling Jaya, Selangor, Malaysia |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.





The information shared in this Journal is really very useful.
I want to know
1. Is this any limitation to submit number of variations for one product with Malaysia health agency for Biological products?
2. Is there any pre-submission meeting with health agency required before filling variations?