Barriers to expanding biosimilars in oncological indications in Chile: A value-chain approach to understand visions and propose recommendations for improving value proposition
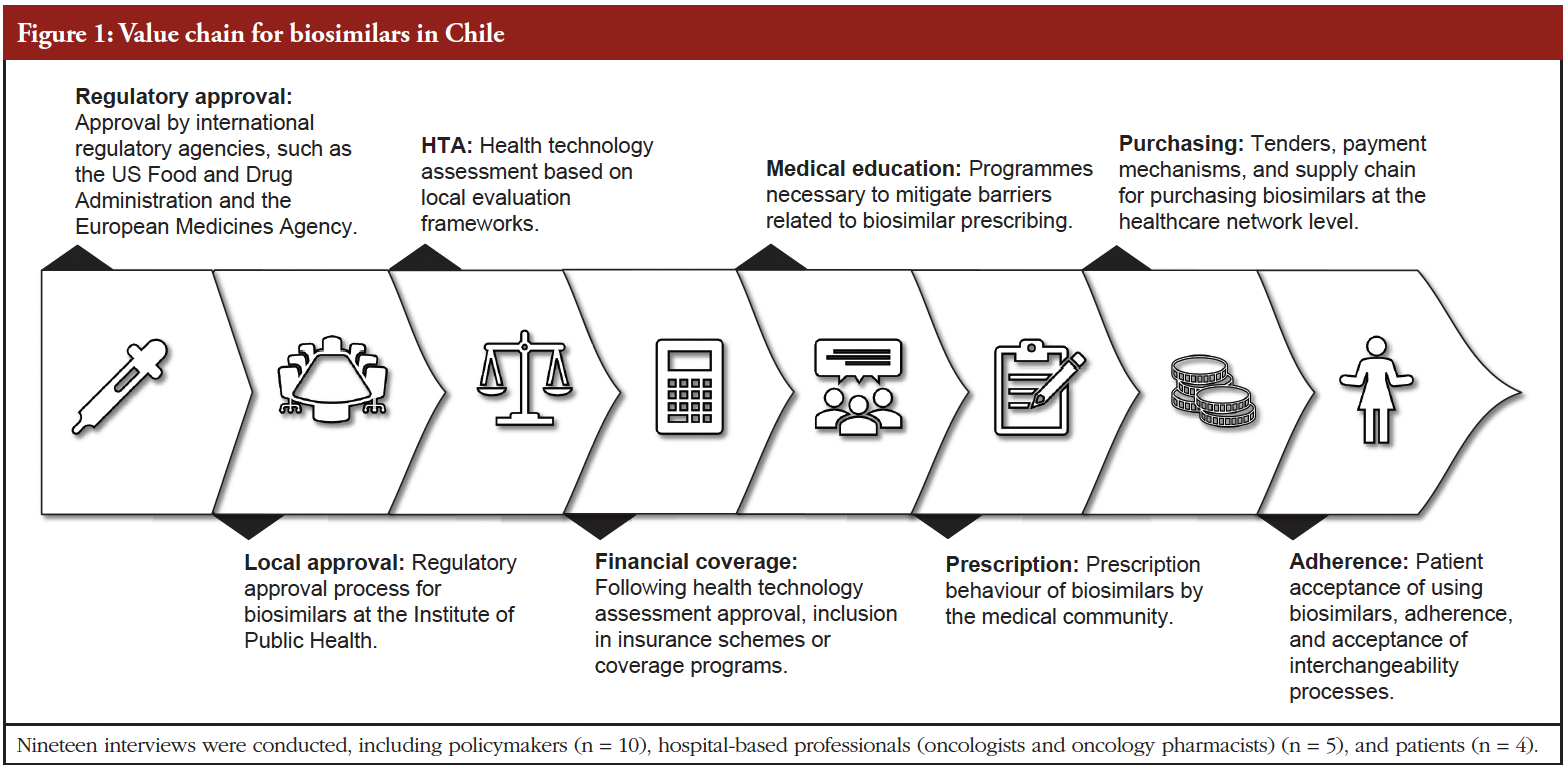
Introduction and Study Objectives: Despite their well-established potential to reduce healthcare expenditures, biosimilars have not achieved widespread adoption in Chile, particularly in oncology. The lack of regulatory incentives and reimbursement frameworks only partially explains this phenomenon. This study aims to identify key barriers to biosimilar adoption in the Chilean healthcare system and propose strategic recommendations […]


