An overview of the current status of follow-on biologicals in Iran
Published on 2020/11/30
Generics and Biosimilars Initiative Journal (GaBI Journal). 2021;10(2):100-6.
Author byline as per print journal: Farhang Rezaei, PharmD; Nassim Anjidani, PharmD
|
Background: The advent of follow-on biologicals in Iran and biosimilars worldwide have provided various treatment options for several severe and chronic diseases. The goal of the present study was to provide an overview of their current status in Iran. |
Submitted: 5 September 2020; Revised: 13 January 2021 ;Accepted: 13 January 2021; Published online first: 29 January 2021
Introduction
Since the development of human insulin via recombinant DNA technology in 1982, several medicinal biotechnological therapeutics have been marketed. These products have broadened the treatment armament for use against life-threatening and chronic diseases [1]. Due to costly manufacturing processes and the considerable investment in research and development required prior to their approval, these products are generally high priced. The mean daily cost of treatment with biotechnological therapeutics is estimated to be 22 times higher than conventional small-molecule medicines [2]. Therefore, despite biologicals’ well-known benefits, including providing more specific and life-saving treatments for a variety of diseases, their use will be restricted by ever-increasing healthcare costs. Healthcare authorities have been eager to find more affordable choices to facilitate patients’ access to these therapies. Follow-on biologicals are potentially less expensive options that can deliver the same benefits of originator biotechnological therapeutics.
According to the US Food and Drug Administration (FDA) definition, a biosimilar is a biological product that is highly similar to an already-approved biological with no clinically meaningful differences from the originator [3]. The European Medicines Agency (EMA) was the first regulatory agency to lay down a biosimilar approval framework in 2005 [4]. Following this, in 2006, somatropin became the first biosimilar molecule that was granted European Union (EU) marketing authorization. Since then, several biosimilar products have been launched in Europe. In 2013, an infliximab biosimilar was the first biosimilar monoclonal antibody that received EMA approval. Due to the complex macromolecular structure of biologicals and their manufacturing procedures’ sensitivity to small variations, approval frameworks outline the rigorous quality control and quality assurance measures required to ensure biosimilar and reference products’ comparability. In addition, they define when clinical studies are required to demonstrate that a biosimilar product is highly similar to the originator in terms of efficacy and safety [5].
Following the development of EMA’s biosimilar approval framework, the World Health Organization (WHO) and FDA established their own biosimilar authorization pathways [6, 7]. According to the guidelines published by EMA, FDA and WHO, several countries have established country-specific guidelines for biosimilar approval [8]. The Iranian pharmaceutical regulatory authority (Iran Food and Drug Administration, IFDA) developed its national biosimilar guidance based on WHO guidelines in 2010 [9].
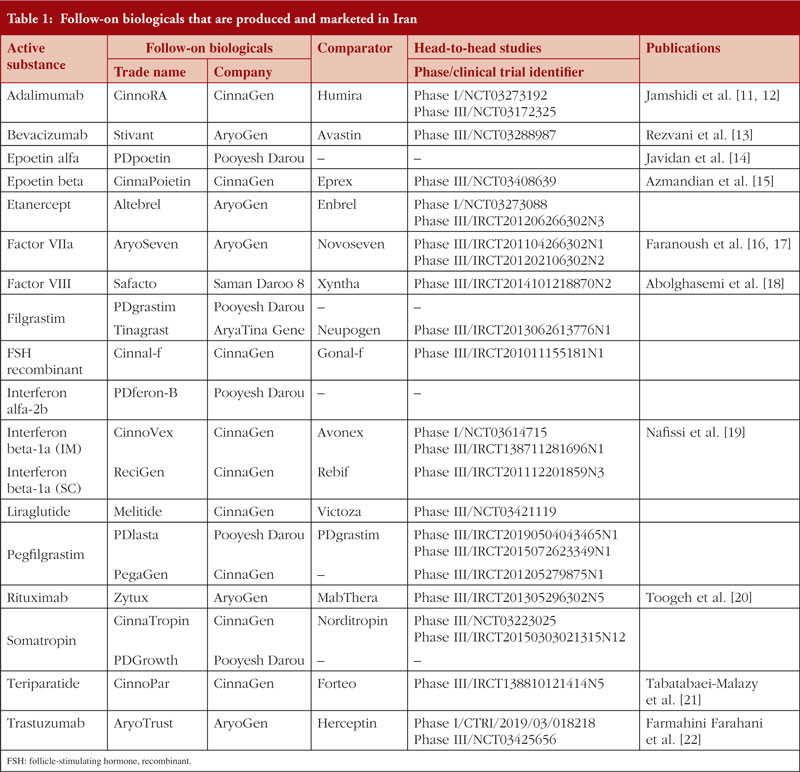
The pharmaceutical industry in Iran began over 80 years ago [10]. In recent years, the commercial potential of producing follow-on biologicals and the favourable economic returns they offer, have encouraged several science-based companies to move towards producing biotechnological therapeutics. In 2000, Interferon alfa-2b was the first follow-on biological that was marketed in Iran. Since then, several other products have been developed and marketed in the country. This product, and other follow-on biologicals that were marketed before the issuance of IFDA biosimilar guidance in 2010, cannot be called biosimilars. This is because many of these follow-on products have not registered or published head-to-head clinical trials with the reference product and have not demonstrated that they are highly similar to their reference product, see Table 1.
The present study had several goals: (1) To identify the follow-on biologicals in the Iranian market and summarize the available information regarding their market entry and related clinical trials; (2) To assess the share of biotechnological therapeutics in Iran’s pharmaceutical market and provide an overview of follow-on biologicals’ share in terms of the number of units sold (sales volume), total sales (sales value), and domestic production/imports; (3) To estimate the potential and actualized cost-saving associated with the use of follow-on biologicals; (4) To summarize information regarding follow-on biological candidates that might enter the market in the future.
Methods
Information from the Iranian Registry of Clinical Trials (IRCT), clinicaltrials.gov, and the International Clinical Trials Registry Platform (ICTRP) was used to identify related phase I and III clinical trials between 2013 and 2018. A PubMed and Google Scholar search was also conducted to find related scholarly articles. The descriptive search terms selected to retrieve data from online sources included the brand name, company name, generic name, biosimilar, clinical trial registration identifier, and Iran. The basic Boolean operator “AND” was used to combine the search terms in various ways in an effort to narrow down search results. Several articles were found using the snowball method by referring to article’s bibliography to pinpoint other relevant articles. In addition, information regarding marketing approval date, brand name and pipeline products of the companies were gathered through referring to reliable pharmaceutical, organizational and governmental sites, as well as via Google search in both English and Persian.
The information regarding pharmaceutical products sales was obtained from IFDA database. This database, which is updated annually, compiles information from all the pharmaceutical wholesale and distribution companies in Iran. The information includes the generic and brand name, dosage form, administration route, producer company, sales volume, and value. The proportion of healthcare spending on biotechnological therapeutics was calculated by dividing total sales of biotechnological products by the total sales of pharmaceutical products in Iran. By identifying the originators and follow-on biologicals for each molecule in the database, the total number of units sold (sales volume) and the total sales (sales value) of biotechnological products were obtained for follow-on biologicals and originators. Also, by determining the finished product producer, the share of domestic production and imported products was calculated in terms of sales volume and sales value. The compound annual growth rate (CAGR) was calculated during the 2013–2018 period. Since most biotechnological therapeutics were available in different potencies, the number of units sold was converted into a specific potency for each molecule (unified sales volume). The unit price was obtained by dividing the sales value by sales volume for each product. All the sales values and unit prices were converted into United States Dollar (USD), based on the average annual exchange rates provided in the customs administration of Iran (IRICA) website.
The actualized and potential cost savings associated with using follow-on biologicals were calculated using two scenarios. The actualized cost saving was determined by subtracting the total sales value of the molecule (originator and biosimilar/follow-on biological) from the product of multiplication of total sales volume of the molecule (originator and biosimilar/follow-on biological) in originator’s unit price. The potential cost saving that could be realized by using follow-on biologicals instead of originators was calculated by subtracting the product of the sales volume of originators in the unit price of the biosimilar/follow-on biological by year (if such an option was available that year) from the product of total sales volume (originator and biosimilar/follow-on biological) in the originator’s unit price.
The quantitative data were analysed using Microsoft Excel 2016 (Microsoft Corporation, USA).
Results
At the time of writing, 21 follow-on biologicals are produced and marketed in Iran, see Figure 1.
These products are related to 17 different reference products. Information regarding these follow-on biological products, including details of head-to-head studies with reference products, and their related publications, is summarized in Table 1.
The total biologicals market in Iran reached approximately US$745 million in 2018 (6-year CAGR = 21.51%). The proportion of healthcare spending on biologicals was 13.5% in 2018 (5-year CAGR = 1.38%).
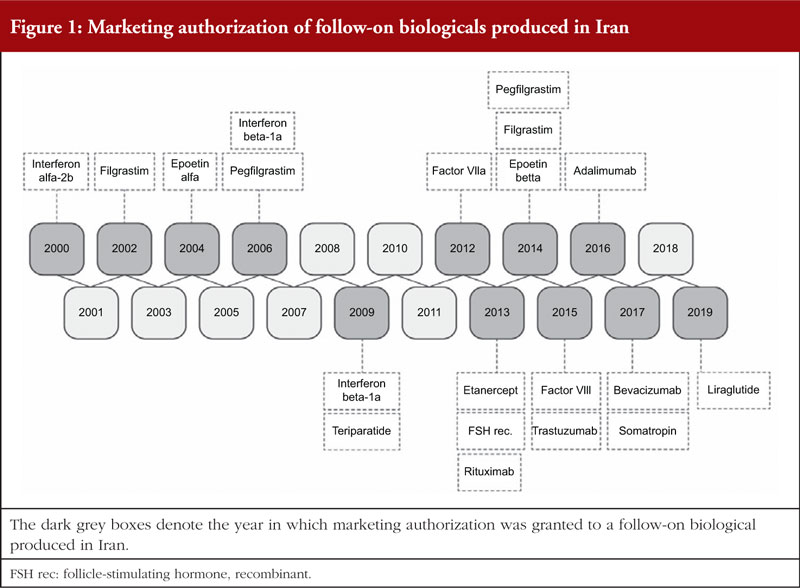
In 2018, follow-on biologicals comprised approximately 47.2% of the total sales value of biotechnological therapeutics, up from 35.2% in 2013 (6-year CAGR = 28.6%). Despite demonstrating an upward trend (6-year CAGR = 16.64%), the proportional annual sales value of originators compared to the total sales value of biotechnological therapeutics decreased from 64.8% in 2013 to 52.8% in 2018, see Figure 2A. Conversely, in terms of the sales volume (number of units sold) the 6-year CAGR of follow-on biologicals (15.74%) was lower than the originators (43.49%). The proportional sales volume of follow-on biologicals in 2018 decreased to 34.6% from 60.7% in 2013. While originators proportional sales volume reached to 65.5% in 2018 up from 39.3% in 2013, see Figure 2B.
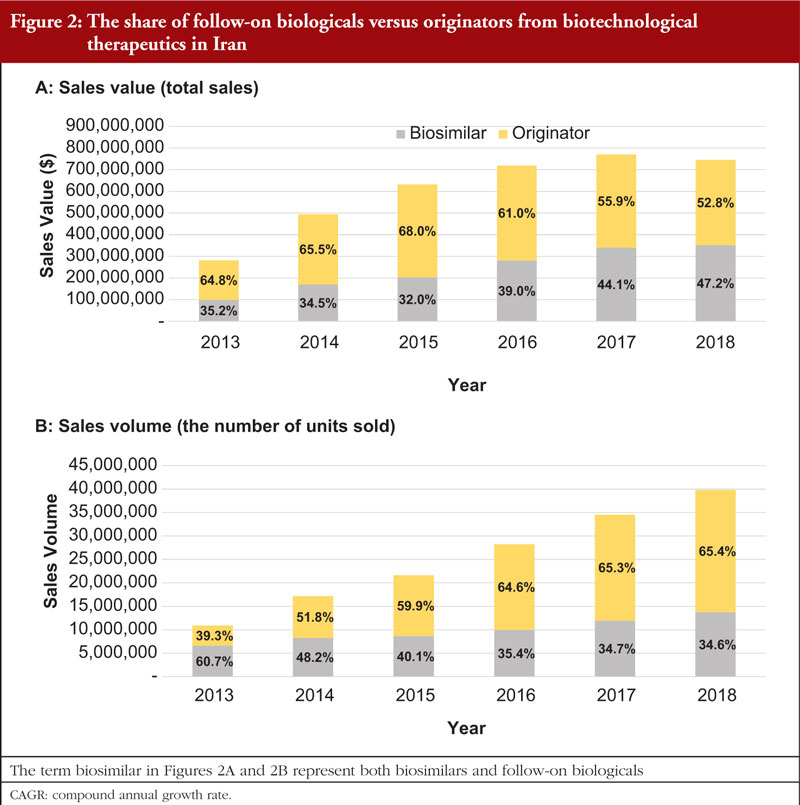
As is the case for originator products, some follow-on biologicals used in Iran are imported from other countries. Figure 3 represents the proportional share of the domestic production or import of the biotechnological therapeutics in Iran, in terms of sales value, see Figure 3A and sales volume, see Figure 3B. In 2018, 42.5% of the follow-on biologicals in the Iranian pharmaceutical market were produced domestically, up from 27.8% in 2013 (6-year CAGR = 28.86%). The proportional sales value of the imported products decreased from 72.2% in 2013 to 57.5% in 2018, despite having a positive 6-year CAGR of 16.64%, see Figure 3A. The 6-year CAGR of domestic products (15.29%) was lower than that for imported products (40.56%) with respect to sales volume. The proportional sales volume of domestic products declined from 53.2% in 2013 to 29.7% in 2018. While the proportional sales volume of the imported products reached 70.3% in 2018, up from 39.3% in 2013, see Figure 3B.
From 2013 to 2018, IFDA priced the follow-on biologicals on average at 54.3±17.8% (mean ± standard deviation) of the originators’ price. during this time, the price of follow-on biologicals was 12%–78% lower than the originators.
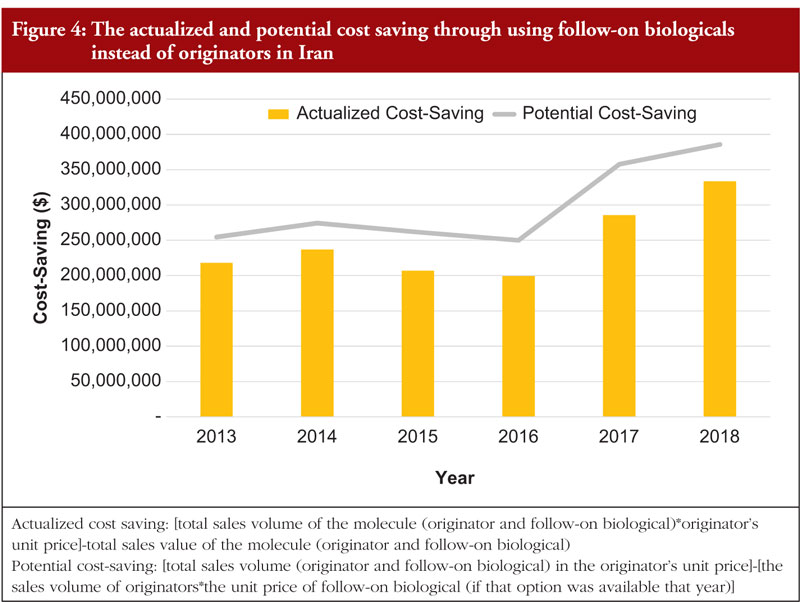
The actualized cost saving associated with using follow-on biologicals has increased in recent years, with a 6-year CAGR of 8.91%, see Figure 4. The significant increase in cost saving in 2017 and 2018 has been mainly driven by an increase in the use of rituximab, adalimumab and trastuzumab follow-on biologicals. The potential cost-saving associated with using follow-on biologicals is also shown on Figure 4. The proportion of actualized cost saving to the potential cost saving was 82.68% on average. This means that it is possible to increase the uptake of follow-on biologicals and benefit from their associated cost savings.
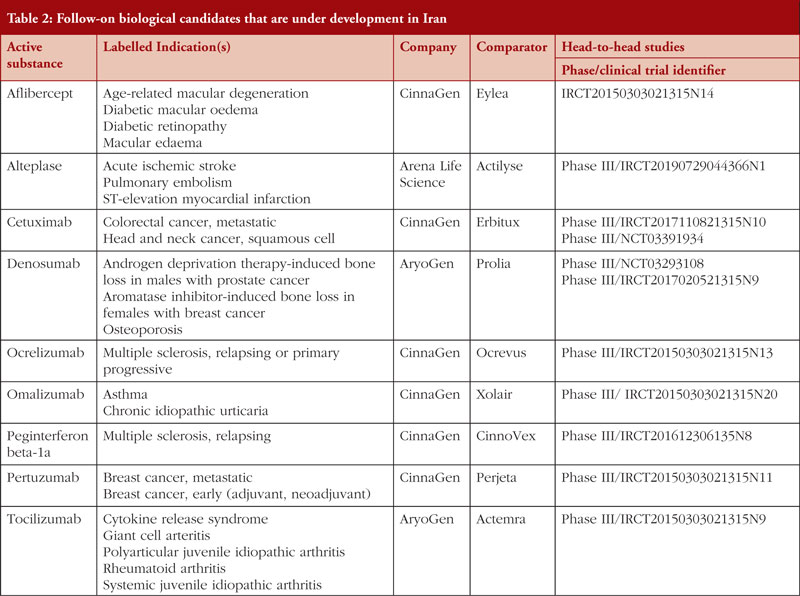
As summarized in Table 2, several biosimilar candidates are under development in Iran. They may be granted marketing authorization in the future if they provide satisfactory comparative efficacy and safety results. These are biosimilar candidates for aflibercept, alteplase, cetuximab, denosumab, ocrelizumab, omalizumab, peginterferon beta-1a, pertuzumab, and tocilizumab. These biosimilar candidates are mostly monoclonal antibodies used to treat several life-threatening and debilitating conditions.
Discussion
In the present study, the current status of follow-on biologicals in the Iranian market has been investigated by evaluating their ongoing and completed clinical trials. The article has highlighted that, as some products were approved as follow-on biologicals prior to the issuance of IFDA biosimilar guidance, they have not registered or published head-to-head clinical trials against the reference product. As biosimilars offer exciting therapeutic opportunities for healthcare authorities and patients in Iran in the future, all efforts should be made to ensure that true biosimilars are approved that are highly similar to reference products in terms of quality, efficacy and safety.
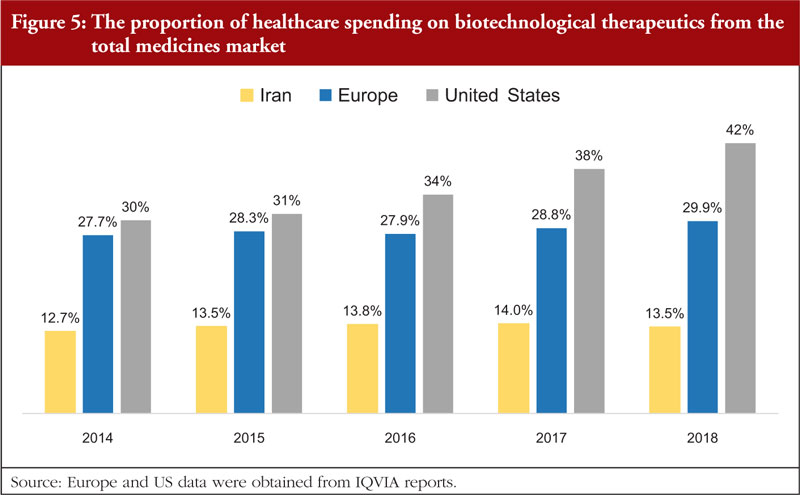
The annual national wholesale data on pharmaceutical products suggests that the use of biologicals in Iran has increased considerably in recent years in terms of both sales value and sales volume. However, there was only a slight increase in the proportional share of biotechnological therapeutics with respect to the total medicines market (from 12.7% in 2013 to 13.5% in 2018). According to the IQVIA reports, in 2018, biologicals represented 29.9% of the total medicines market in Europe with a CAGR of 1.93%. In the US, 42% of healthcare costs for medicines were devoted to biotechnological therapeutics (5-year CAGR = 8.78%) [23, 24]. Despite an increasing trend in the use of biological therapeutics in Iran, the proportion of healthcare expenditure on biotechnological therapeutics here is considerably lower than in Europe and the US, see Figure 5. The unwillingness of the Iranian healthcare authorities to add new molecules, specifically biotechnological therapeutics, to Iran’s national drug list may be a contributing factor to the comparatively low uptake of these products. This issue may stem from the greater cost associated with using biotechnological therapeutics and connected increase in healthcare burden. The importance of biotechnological therapeutics in the treatment of a wide range of severe and chronic diseases, together with escalating healthcare costs highlights the importance of using biosimilars as a lower-priced substitute for originator biologicals. The use of biosimilars can significantly reduce healthcare expenditure on biotechnological therapeutics. According to an evaluation by Mulchay et al. [25], it was projected that the emergence of biosimilars would reduce US healthcare spending by US$54 billion in 2026. The proportional difference in the use of biotechnological therapeutics in Iran compared to other regions might also be explained by various different pricing strategies adopted for biosimilars and follow-on biologicals. For example, in Europe, biosimilar developing companies have to price their products at least 30% lower than the originator [4]. According to a recent review of literature, the price difference between biosimilar developing companies and their originators ranges between 15%–30% [4]. While in Iran, from 2013 to 2018, follow-on biologicals’ price has been approximately 54.3% of originators.
In recent years, the share of follow-on biologicals produced domestically has increased considerably in terms of sales value. At the same time, the trend of the increase in sales volume was moderate compared to sales value. This might suggest that pharmaceutical and biotechnology companies in Iran have been more willing to invest in high-value, low-volume production. This tendency is also apparent in the biosimilar candidates in the companies’ pipelines. The majority of these products are monoclonal antibodies that usually have a high unit price.
The introduction of follow-on biologicals has offered significant cost saving for the Iranian healthcare system as actualized cost saving through follow-on biologicals use reached over US$300 million in 2018. These savings were influenced by the rate of follow-on biologicals use and the difference in unit price between follow-on biologicals and originators. Rituximab, adalimumab and trastuzumab were the main drivers of the significant cost saving in 2017 and 2018. This figure is likely to increase in the upcoming years due to the market entry of other biosimilars/follow-on biologicals currently under development.
The pharmaceutical landscape of Iran has changed significantly in the past 20 years. In recent years, some biopharmaceutical companies in Iran (CinnaGen and AryoGen) have endeavoured to move towards regulated markets, such as Europe. As a preliminary step, they have acquired EU-good manufacturing practice (GMP), the certificate of GMP, that has been published on EMA’s database [6, 7]. In 2017, CinnaGen initiated pharmacokinetics (PK) and pharmacodynamics (PD) studies of its interferon beta-1a biosimilar (CinnoVex®) in Finland [8]. As such, it seems that by the development of high-quality biosimilars bolstered by satisfactory non-clinical and clinical studies, Iranian pharmaceutical companies can expand their presence in regulated and semi-regulated markets.
Conclusion
Although biotechnological therapeutics have offered life-saving therapies to many critical diseases, their proportional uptake in Iran has remained constant in recent years. It seems that healthcare authorities can improve patients’ access to these medicines through promoting the use of biosimilars/follow-on biologicals instead of costly originators once their quality, safety, and efficacy have been ensured. The significant cost saving associated with using biosimilars/follow-on biologicals can be allocated to helping new biotechnological medicines to be included in Iran’s drug list.
Competing interests: Farhang Rezaei and Nassim Anjidani work in the medical department of Orchid Pharmed company which is in collaboration with AryoGen Pharmed and CinnaGen companies with respect to conducting clinical trials.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Farhang Rezaei, PharmD
Nassim Anjidani, PharmD
Medical Department, Orchid Pharmed Company, No 42. Attar Streeet, North Kurdistan Highway, 1994766411 Tehran, Iran
References
1. Espiritu MJ, Collier AC, Bingham J-P. A 21st-century approach to age-old problems: the ascension of biologics in clinical therapeutics. Drug Discov Today. 2014;19(8):1109-13.
2. Cheraghali AM. Biosimilars; a unique opportunity for Iran national health sector and national pharmaceutical industry. Daru. 2012;20(1):35.
3. U.S. Food and Drug Administration. Biosimilar and interchangeable products. 2017 [homepage on the Internet]. [cited 2021 Jan 13]. Available from: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products
4. Kabir ER, Moreino SS, Sharif Siam MK. The breakthrough of biosimilars: a twist in the narrative of biological therapy. Biomolecules. 2019;9(9):410.
5. O’Callaghan J, Barry SP, Bermingham M, Morris JM, Griffin BT. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol. 2019;75(1):1-11.
6. GaBI Online – Generics and Biosimilars Initiative. MENA region biologicals maker CinnaGen receives EU GMP certification [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Jan 13]. Available from: http://gabionline.net/Pharma-News/MENA-region-biologicals-maker-CinnaGen-receives-EU-GMP-certification
7. GaBI Online – Generics and Biosimilars Initiative. MENA mAb manufacturer AryoGen receives EU GMP approval [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Jan 13]. Available from: http://gabionline.net/Pharma-News/MENA-mAb-manufacturer-AryoGen-receives-EU-GMP-approval
8. Cinnagen. Comparative trial of the pharmacokinetics and pharmacodynamics of intramuscularly injected CinnoVex® and Avonex® in healthy volunteers clinicaltrials.gov; 2018. Report No.: NCT03614715 [homepage on the Internet]. [cited 2021 Jan 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT03614715
9. The Iranian national biosimilar guidance [homepage on the Internet]. [cited 2021 Jan 13]. Available from: http://fdo.behdasht.gov.ir/index.aspx?siteid=114&pageid=40850&siteid=114
10. Majid Cheraghali A. Current status of biopharmaceuticals in Iran’s pharmaceutical market. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):26-9. doi:10.5639/gabij.2013.0201.008
11. Jamshidi A, Gharibdoost F, Vojdanian M, Soroosh SG, Soroush M, Ahmadzadeh A, et al. A phase III, randomized, two-armed, double-blind, parallel, active controlled, and non-inferiority clinical trial to compare efficacy and safety of biosimilar adalimumab (CinnoRA®) to the reference product (Humira®) in patients with active rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):168.
12. Jamshidi A, Sabzvari A, Anjidani N, Shahpari R, Badri N. A randomized phase I pharmacokinetic trial comparing the potential biosimilar adalimumab (CinnoRA®) with the reference product (Humira®) in healthy volunteers. Expert Opin Investig Drugs. 2020;29(3):327-31.
13. Rezvani H, Mortazavizadeh SM, Allahyari A, Nekuee A, Najafi SN, Vahidfar M, et al. Efficacy and safety of proposed bevacizumab biosimilar BE1040V in patients with metastatic colorectal cancer: a phase III, randomized, double-blind, noninferiority clinical trial. Clinical Ther. 2020;42:848-59.
14. Norouzi Javidan A, Shahbazian H, Emami A, Yekaninejad MS, Emami-Razavi H, Farhadkhani M, et al. Safety and efficacy of PDpoetin for management of anemia in patients with end stage renal disease on maintenance hemodialysis: results from a phase IV clinical trial. Hematol Rep. 2014;6(3):5195.
15. Azmandian J, Abbasi MR, Pourfarziani V, Nasiri AA, Ossareh S, Ezzatzadegan Jahromi S, et al. Comparing therapeutic efficacy and safety of epoetin beta and epoetin alfa in the treatment of anemia in end-stage renal disease hemodialysis patients. Am J Nephrol. 2018;48(4):251-9.
16. Faranoush M, Abolghasemi H, Toogeh Gh, Karimi M, Eshghi P, Managhchi M, et al. A comparison between recombinant activated factor VII (Aryoseven) and Novoseven in patients with congenital factor VII deficiency. Clin Appl Thromb Hemost. 2015;21(8):724-8.
17. Faranoush M, Abolghasemi H, Mahboudi F, Toogeh G, Karimi M, Eshghi P, et al. A comparison of efficacy between recombinant activated factor VII (Aryoseven) and Novoseven in patients with hereditary FVIII deficiency with inhibitor. Clin Appl Thromb Hemost. 2016;22(2):184-90.
18. Abolghasemi H, Panahi Y, Ahmadinejad M, Toogeh G, Karimi M, Eghbali A, et al. Comparative evaluation of the safety and efficacy of recombinant FVIII in severe hemophilia A patients. J Pharmacopuncture. 2018;21(2):76-81.
19. Nafissi S, Azimi A, Amini-Harandi A, Salami S, Shahkarami MA, Heshmat R. Comparing efficacy and side effects of a weekly intramuscular biogeneric/biosimilar interferon beta-1a with Avonex in relapsing remitting multiple sclerosis: A double blind randomized clinical trial. Clin Neurol Neurosurg. 2012;114(7):986-9.
20. Toogeh G, Faranoush M, Razavi SM, Jalaeikhoo H, Ravanbod MR, Zarrabi F, et al. A double-blind, randomized comparison study between ZytuxTM vs MabThera® in treatment of CLL with FCR Regimen: non-inferiority clinical trial. Int J Hematol Oncol Stem Cell Res. 2018;12(2):84-91.
21. Tabatabaei-Malazy O, Norani M, Heshmat R, Qorbani M, Vosoogh A, Afrashteh B, et al. Efficacy and safety of the biosimilar recombinant human parathyroid hormone Cinnopar® in postmenopausal osteoporotic women: a randomized double-blind clinical trial. Iran J Public Health. 2018;47(9):1336-44.
22. Farahani MF, Maghzi P, Aryan NJ, Payandemehr B, Soni M, Azhdarzadeh M. A randomized, double-blind, parallel pharmacokinetic study comparing the trastuzumab biosimilar candidate, AryoTrust®, and reference trastuzumab in healthy subjects. Expert Opin Investig Drugs. 2020;29(12):1443-50.
23. Troein P, Newton M, Patel J, Scott K. The impact of biosimilar competition in Europe. IQVIA. 2019.
24. Aitken M. Biologics market dynamics: setting the stage for biosimilars. IQVIA Institute for Human Data Science. 2020.
25. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States. Rand Health Q. 2018;7(4):3.
|
Author for correspondence: Farhang Rezaei, PharmD, Medical Department, Orchid Pharmed Company, No. 42, Attar Street, Attar Square, North Kurdistan Highway, 1994766411 Tehran, Iran |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2021 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.