Switching from the infliximab reference product to CT-P13 in patients with rheumatoid arthritis or ankylosing spondylitis: results of the PLANETAS and PLANETRA extension studies
Published on 2016/06/27
Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(2):94-5.
Submitted: 24 May 2016; Revised: 8 June 2016; Accepted: 10 June 2016; Published online first: 24 June 2016
CT-P13, also known by its brand names Remsima or Inflectra, is a biosimilar of the infliximab reference product (RP) Remicade. CT-P13 is approved in Europe, the US and elsewhere for use in all indications for which the RP is licensed, including rheumatoid arthritis (RA) and ankylosing spondylitis (AS). Approval of CT-P13 was partly based on findings from two pivotal clinical trials – PLANETAS and PLANETRA – which concluded that CT-P13 and the RP were highly comparable in terms of pharmacokinetics, efficacy, immunogenicity and safety in AS patients (PLANETAS) and RA patients (PLANETRA). Recently, data from extension phases of the original two clinical trials have been published in Annals of the Rheumatic Diseases. The data reported that it was possible to switch from the RP to CT-P13 without any detrimental effects on safety or efficacy. The studies also reported that CT-P13 was well tolerated and effective for up to two years in patients with RA and AS [1, 2].
Following the main 54-week randomized controlled trials during which patients with AS or RA received either the RP or CT-P13 [3, 4], patients were invited to enrol into 48-week extension studies. All patients who enrolled onto the extension studies were treated with CT-P13. Clinical efficacy and disease activity were assessed using a variety of different measurements, including Assessment of SpondyloArthritis international Society (ASAS) response rates (in patients with AS) and American College of Rheumatology (ACR) response rates (in patients with RA).
Three hundred and two patients with RA were enrolled and 301 were treated with CT-P13 in the PLANETRA extension study. Of these, 158 had received CT-P13 in the original study (‘maintenance group’) and 144 had received the RP (‘switch group’). One hundred and seventy-four patients with AS were treated in the PLANETAS extension study. Of these, 88 had received CT-P13 in the original study (‘maintenance group’) and 86 had received the RP (‘switch group’).
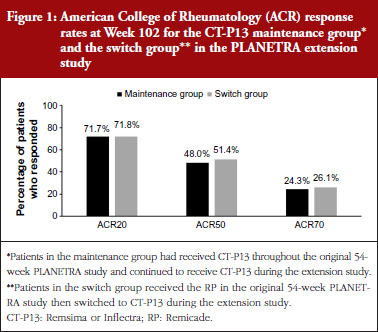
In both extension studies, clinical efficacy was similar in patients in the maintenance and switch groups. In RA patients, ACR20, ACR50 and ACR70 rates were not different between the maintenance and switch groups, see Figure 1. In AS patients, ASAS20, ASAS40 and ASAS partial remission rates were also similar between the two groups, see Figure 2.
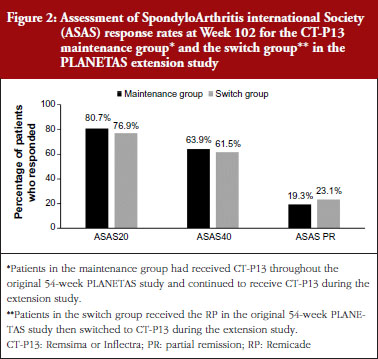
Clinical efficacy was maintained over 2 years in the patients with RA and AS who had received CT-P13 during both the original and extension studies. In patients with RA, the ACR20 response rate was 77.0% after 1 year of treatment with CT-P13, and 71.7% after 2 years of treatment. In patients with AS, the ASAS20 response rate was 70.5% after 1 year of treatment with CT-P13, and 80.7% after 2 years of treatment.
CT-P13 was well-tolerated during both extension studies, with a safety profile that was consistent with that of the RP [5, 6]. The proportion of patients developing anti-drug antibodies was also similar in the maintenance and switch groups in either extension study.
As patent expiration dates loom for many biological drugs, there is increasing interest in the development of biosimilar drugs. With tight healthcare budgets, many countries commonly recommend the least expensive drug be used for treating conditions such as RA and AS [7, 8]. With biosimilars typically being less expensive than originator biologicals [9], studies that examine whether patients can be switched from the originator biological to a biosimilar are vital to aid clinical decision-making. Switching to the biosimilar may lead to cost savings and increased access to treatment for more patients with these debilitating diseases.
Competing interests: The authors of research papers [1, 2] declared that DHY and WP are consultants for Celltrion; for full details of all authors’ conflicts of interest, see the research papers [1, 2].
Provenance and peer review: Article abstracted based on published scientific or research papers [1, 2]; internally peer reviewed.
Editorial support for the article abstract of Professors Won Park and Dae Hyun Yoo was provided by Philippa Flemming, PhD, funded by Celltrion Healthcare Co Ltd.
References
1. Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. doi:10.1136/annrheumdis-2015-208786
2. Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitits: 102-week data from the PLANETAS extension study. Ann Rheum Dis. doi:10.1136/annrheumdis-2015-208783. Epub ahead of print
3. Yoo DH, Racewicz A, Brzwzicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016;18:82. doi:10.1186/s13075-016-0981-6
4. Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
5. Braun J, Deodhar A, Dijkmans B, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum. 2008;59(9):1270-8.
6. Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50(4):1051-65.
7. National Institute for Health and Care Excellence. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. NICE technology appraisal guidance (TA375). 26 January 2016 [homepage on the Internet]. [cited 2016 Jun 8]. Available from: https://www.nice.org.uk/guidance/TA375/chapter/1-Recommendations.
8. National Institute for Health and Care Excellence. TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis. NICE technology appraisal guidance (TA383). 01 February 2016 [homepage on the Internet]. [cited 2016 Jun 8]. Available from: https://www.nice.org.uk/guidance/ta383.
9. IMS Health. Shaping the biosimilars opportunity: a global perspective on the evolving biosimilars landscape. December 2011 [homepage on the Internet]. [cited 2016 Jun 8]. Available from: http://weinberggroup.com/pdfs/Shaping_the_biosimiliars_opportunity_A_global_perspective_on_the_evolving_biosimiliars_landscape.pdf
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2016 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.