The refinement of the super generic concept: semantic challenge for product re-innovation?
Published on 2015/03/26
Generics and Biosimilars Initiative Journal (GaBI Journal). 2015;4(1):25-32.
|
Background: Uptake of super generic or hybrid pharmaceuticals has decelerated despite their important economic potential for the generic pharmaceutical industry. The aim of switching to these product portfolios was to enable product differentiation; however, these strategies are influenced by new semantic challenges, which have hampered the promotion of value-added pharmaceuticals or super generics in recent years. |
Submitted: 2 October 2014; Revised: 25 February 2015; Accepted: 10 March 2015; Published online first: 20 March 2015
Introduction
Previous studies comparing brand-name drugs with generic drugs have focused on cost-reduction rather than clinical outcome. The focus of this study is on the improved product itself and how this improvement contributes to promotion of the product. Improvements can include a new dosage form, a route of administration, a new indication, or a combination of these. In such cases, the product cannot be considered as a simple generic. For such a product, new data supporting any new features must be generated.
In the US, drugs approved under 505(b)(2) can rely, in part, on data from existing reference drugs. This means that they can be developed and achieve US Food and Drug Administration (FDA) approval in as little as 30 months, with only a fraction of the number of required clinical trials and at much lower cost. Additionally, unlike generic drugs approved under section 505(j), in which exclusivity can be held for only 180 days, the 505(b)(2) applicant may qualify for 3, 5, or 7 years of market exclusivity, depending on the extent of the change to the previously approved drug and the type of clinical data included in the new drug application (NDA).
According to some of the producers interviewed, using 505(b)(2) as the foundation of a drug development programme is a fast and cost-effective strategy that has proved its functionality. An alternative to spending important sums on developing blockbusters is to concentrate on developing products for niche markets that may have smaller market potential, but that can be approached with dramatically lower development costs using the section 505(b)(2) pathway for FDA approval [1].
Given that the large pharmaceutical companies are developing ‘fewer’ innovative small molecules, the decrease in innovative products results in fewer generic drug targets and challenges the sustainability of the generics industry. The importance of developing products via 505(b)(2) has motivated generic pharmaceutical manufacturers to find solutions for product differentiation and sustainability of this industry.
Furthermore, an evolution in nomenclature for differentiated generic drug products has been observed in the past four years.
In the present study of terminology used in generic pharmaceutical innovation, we aimed to assess the possibility of real ‘product innovation’ and how generics companies could organize this ‘innovative’ activity.
In the intervening years, it seems that little progress has been made by pure generic drug companies in this area. We question whether the inclusion of the term ‘generic’ in super generic or value-added generic may in fact partially contribute to this lack of success because, intuitively, the terms ‘generic’ and ‘innovative’, may appear to be oxymoronic.
In this study, we analyse the change in usage of terms such as super generics, value-added generics, new therapeutic entities, and premium generics.
Methods
Data collection
A qualitative approach was taken for this study. A semi-structured format was selected because structured interviews often produce quantitative data. Semi-structured interviews are generally organized around a set of predetermined open-ended questions, with other questions emerging from the dialogue between interviewer and interviewee. This iterative process of data collection and analysis eventually leads to a point in the data collection at which no new categories or themes emerge. This is referred to as saturation, signalling that data collection is complete.
Semi-structured interviews were conducted with managers, industry consultants, lawyers and researchers to collate their views and perspectives, and to enable their experiences in this area to be shared. Between April 2013 and June 2014, 10 interviews were conducted (the number of people who agreed to be interviewed). The length of interviews varied between 45 and 90 minutes. They were conducted in Europe, India and USA.
The interviews were conducted according to a predefined questionnaire; however, new topics would sometimes emerge during the interviews. The questionnaire focused specifically on ‘super generic’ products and how semantic alternatives may influence marketing strategy. The questionnaire was arranged into two parts: a survey about the history and background of super generics and a questionnaire about the evolution of the use of super generics as product types in published research. Questions in parts one and two of the questionnaire are presented in Table 1.
The semi-structured interviews enabled new and up-to-date information to be collected on changes in the super generic phenomenon, thereby filling knowledge gaps in published articles and reports. They were useful for opening discussions with professionals and obtaining different versions of this story. Such details could not be easily discovered by using a non-qualitative method. Qualitative research data are based on human experience, and are therefore powerful and sometimes more compelling than quantitative data. Subtleties and complexities about the research subjects, topic, or both, are often discovered that are sometimes missed by other forms of enquiries.
‘Open-ended’ questions are not necessarily worded in exactly the same way with each participant. Participants are free to respond in their own words, and these responses tend to be more complex than simply ‘yes’ or ‘no’ answers used in quantitative methods.
The same procedures in interview and data collection were used as the first article of this series about the strategic importance of super generic pharmaceutical and the new era for the generics pharmaceutical industry.
Interviewees were classified by an extract of their name in our node list. This kept their identity anonymous and is a quick and simple way to label the interview contents.
On the platform of professional groups on LinkedIn, professionals in the pharmaceutical industry were identified, and debates and discussions related to current changes in marketing super generics were initiated.
A literature review was conducted using databases, such as MEDLINE, PUBMED, Google Scholar and other research engines, to collect the relevant articles published in English, French and German between 2004 and 2014. To obtain better results, other published articles and reports related to the subject were also investigated in our literature review, for instance, the IMS reports. The search strategy was developed using 11 search terms [2] relevant to semantic challenges and generic pharmaceutical product re-innovation. The following search terms were used: ‘innovative generic medicines’, ‘super generic pharmaceuticals’, ‘premium generics’, ‘specialty generics’, ‘value-added generics’, ‘new therapeutic entities’, ‘improved therapeutics’, ‘pricing strategy’, ‘marketing strategy’, ‘drug delivery system’, ‘505(b)(2) approvals’. Inclusion criteria were generic medicines, super generic and value proposition.
More specifically the literature review has enabled us:
- To study the definitions of super generics used in previous research works
- To discover relations between different research results by comparing various investigations
- To identify gaps in knowledge, as well as weaknesses in previous studies
Results
The challenge of post-patent expiration is characterized by high-price competition between generics manufacturers. If companies were to invest in a smaller number of value-added generics rather than a large number of conventional generics, they may obtain better returns on investment.
Despite the efforts of several generic pharmaceutical companies, the product innovation potential of generics manufacturers is not always transparent in pharmaceutical and pharmacoeconomic literature.
In 2004, Kermani [3] pointed to the ‘involvement’ of some generics companies in innovative research and development. In this context, innovation is applauded because of the dynamic it may bring to the market, for further financial benefits it will bring for the manufacturers, and also because ‘the effective drug treatment can lessen the economic burden of major diseases’. Health-economic evaluations are related to these results because, ‘improved treatments’ could provide better disease management, shorter hospitalization rates, reduced mortality, and savings for healthcare systems.
Other authors have acclaimed the importance of value-added generics as a new model [4].
The value-added model contains ‘value’ for the business models of such companies that launch an innovation adventure. Here, our attention is focused on the innovation as a ‘value’ profile for the generic pharmaceutical industry.
According to the economic literature, the innovator is the unusual businessman who is able to combine capital, labour and resources to develop a new product, a new service or a new form of business organization [5, 6].
Product innovation is only one part of the story. Innovative change can occur through improvements in the production process, raw material and intermediate inputs, enhancing the efficiency of the management system, and risk reduction [7].
For innovative generics manufacturers, improved therapeutics and pricing, and the return on investment in innovative activities, is just as important as producing a high-quality product. Is innovation rewarding enough for generics manufacturers? Access to a higher pricing system is important for companies using this strategy. With increased pressure on health funds and Health Maintenance Organizations, it is more difficult for a product to make it onto an approved list or reimbursement programme.
Where a product is presented as a line extension of a branded product, there is tacit assumption of added value (whereas it may simply be a case of life-cycle management related to patent expiry). When a generic drug company presents essentially the same product under the designation of super generic, generic plus or added-value generic, it may well be that the reimbursement barrier is raised or even becomes insurmountable.
For example, Actavis has recently reported a negative recommendation from the FDA Advisory Panel for their fixed-dose combination of Valsartan and Nebivolol [8].
The panel vote was split, with six rejecting and four in favour of the product. This narrowest of margins raises the question of whether the vote might have been different had the application been made by a traditionally recognized innovator company and whether there is a subconscious mindset that assumes that generic companies cannot really add value to existing products. The mechanism of application via an NDA, however, would have been the same. It is also still only a recommendation, which is not binding by FDA.
In the most recent cycle of innovation in the generic pharmaceutical industry more acceptable innovative activity included ‘re-innovated’ products emerging from new technology platforms, change in the managerial mindset, and evolving business models [9]. This innovative character aims to satisfy the patients’ unmet medical needs [10].
Increasing access to new technology platforms can enable generic manufacturers to become more active and find new financial returns on their investments in several therapeutic areas, such as pain therapy, respiratory, oncology, geriatrics, and paediatrics.
Labelling challenges of re-innovated products, reminds us of the difficult discipline of integrating ‘newness’ into the ‘old’ product. The only issue that can save the re-innovated product is a real integration of ‘value’ and the satisfaction of unmet medical needs.
Identifying this value in improved generics has become a real marketing strategy in recent years.
Labelling ‘re-innovated’ products: the semantic challenge
A semantic shift has been observed in the designation of super generics in the pharmaceutical literature. Labels such as ‘re-innovated’ products, ‘value-added generics’, ‘new therapeutic entities’, and ‘enhanced therapeutics’, all indicate a subconscious search for a ‘non-generic’ identity and a move away from being simply a ‘generic extension’.
In one of our interviews a pharmaceutical research and development scientist commented:
‘The use of the term super generic, to my view is just a change in the communication strategy (the word super generic has a ‘generic’ component, by eliminating this component you stress the innovation part …’ (2014).
A marketing manager described the issue from another perspective: the absence of a clear product definition and pricing challenges:
‘… I think that the problem is that there is no clear definition of products that are derived from existing drugs/generics; therefore, we see a trend towards the terms used by FDA (505(b)(2) and EMA (Art 10.3). Especially, the pharmaceutical companies want to move away from the term ‘super generics’ as it has the connotation of still being a ‘generic’ and as such it can easily be put under the generic pricing.’(2014).
That is why, in some recent articles, the label ‘super generic’ is avoided because the product in question is a ‘hybrid’ version.
The hopes and expectations related to super generic products are numerous. Apart from economic and financial results that they may generate for the manufacturers, they bring about hope for patients in search of ‘value-added medicines’ that may satisfy their unmet medical needs.
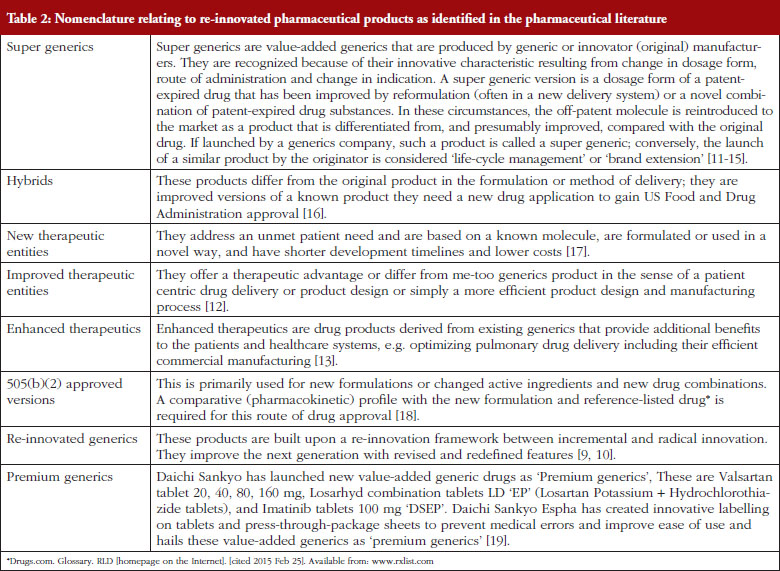
Recently used nomenclature relating to re-innovated pharmaceutical products is presented in Table 2.
The challenge of ‘value’ integration: satisfying ‘great expectations’?
The demand for ‘new medicines’ is increasing. Patients demand ‘new’ but ‘efficient’ and accessible pharmaceuticals; however, solving this equation requires innovation.
The urge of pharmaceutical companies to move away from the terms ‘super generic’ and ‘generic’, indicates that they want to avoid the connotation of still being a ‘generic’ and as such prices can be set at generics levels. That is why Teva brought forward the term ‘novel therapeutic entity’ to indicate the novelty of the product and to align it with the term ‘new chemical entity’.
New therapeutic entities and new chemical entities are also similar.
It seems that all these new labels aim to target new market segmentation and change the concept of ‘new product’. Therefore, ‘value creation’ is reinforced by the gap created between the ‘generic’ and ‘re-innovated’ drug products. In this way, they seek to maximize the expected economic returns at a reduced level of risk in a new product pipeline.
Super generic products may also be seen as linking innovation with affordability [16] for patients. As several versions of a known drug can cause confusion for prescribers and patients, the clear description of the drug category, dosage and means of administration require clear and precise labelling to ensure the prescribers and patients correctly use the innovated dosage form.
‘Innovation’ is taken into account by super generic producers, but it is undoubtedly a complex function of patients’ needs, providers’ capabilities, regulatory frameworks, incentive mechanisms, and intellectual property rights and, of course, the availability of appropriate funding. In the case of re-innovated pharmaceuticals, the ‘value’ proposition to patients and health organizations is the principal business argument with the investment affording an appropriate return. According to Porter (2011): the most important thing about ‘value’ is that it should drive innovation and improve health outcomes.
Furthermore a ‘shared value’ perspective as Porter [20] refers to, focuses on improving, growing techniques and strengthening the local cluster of supporting suppliers and other institutions to increase efficiency, product quality and sustainability. This leads to a bigger part of revenue and profits.
The innovative technology platforms are one of the main tools that can help the pharmaceutical manufacturers to achieve this goal.
Risk reduction and the creation of new market segments are among the targets followed by super generic producers in the search of ‘value creation’; in this framework, even quality by design can be considered as a marketing tool.
‘The market leaders follow the quality by design approach, multifunctional excipients, modifying dosage form, and reforming the release pattern to develop the value added therapies which creates a new segment and uplift the growth in generics market and even assures better quality and economic viability.’
In a more technical discussion, the commercial use of quality by design (QbD) may not have a positive effect on quality, even if the method is scientific and with the intention of providing better results [21].
The commercial use of QbD may divert the industry from taking real innovative initiatives [22]. The quality approach may bring added value and can create ‘value’ itself in the business model but it may be assessed [9].
Quality by design is a concept for planning the quality and to meet customer expectations for ‘value’. The focus of this concept is that quality should be built into a product with a thorough understanding of the product, the process by which it is developed and manufactured, alongside knowledge of the risks involved in the manufacturing of these products and how best to mitigate them.
‘The focus of the QbD exercise is process understanding. One can use many approaches to gain process understanding. The most effective approach is statistically designed experiments. Constructing a design space using any other approach will be costly and provides low-quality models. The greatest challenge is identifying the candidate experimental variables.’
The QbD can be considered as a business argument for developing high quality, low-risk products:
…QbD is perfect as to this aiming (manufacturing of the super generics), especially when the active pharmaceutical ingredient is already available and characterized, pursuing a QbD approach for an improved product. Targeting the specific needs of patients, e.g. ease of ingestion, storage and handling procedures, improved robustness.
If a company simply uses a QbD model as a quality approach, then they are using a ‘strategy’ for making a good product or process design decisions (which would be classified in the area of managerial decisions). The use of quality tools in a structured way improves the business competitive standing by lowering risk and costs in all the dimensions, e.g. regulatory, production, customer value and business.
FDA has fully implemented QbD for abbreviated new drug applications as of January 2013. Manufacturers of generics, therefore, have to pay more attention to quality and adopt QbD.
Patient expectations can be satisfied, either in mature markets or the promising emerging markets, by providing ‘shared values’, see Figure 1.
As generics manufacturers mature and seek ideas for product differentiation, they are becoming more creative and more ‘value-creation’ conscious. In this way, they attempt to move away from short-term financial performance towards the most important customer needs that will determine their long-term success.
Product examples resulting from ‘value-creation’ strategy
The use of new technology platforms can indeed be viewed as a search for value creation and quality product ‘culture’. Examples are discussed below.
The nasal sumatriptan
Sumatriptan allows the same blood levels of the drug to be achieved in 2–3 mins, compared with the currently marketed Imitrex, which takes 60–120 mins [23].
Human clinical trials have shown that the Intravail® formulation of sumatriptan achieves therapeutic drug levels at about 2–3 mins, 20–30 times faster than the currently marketed non-injectable sumatriptan products. For comparison, the most widely used triptan formulations, namely sumatriptan nasal spray or tablet formulations, both reach maximum blood levels of the drug in about 60–120 mins, thus delaying onset of relief. The total triptan market exceeds US$3 billion annually, with sumatriptan comprising approximately US$2 billion of the total.
Chronic respiratory diseases (complex re-innovated product)
According to Schubben et al. [24], pulmonary powder inhalation treatments, such as caperomycin oleate, is superior to sulfate salt and has a favourable risk–benefit profile for the future treatment of tuberculosis. By using more efficient and convenient treatments, the adherence to drug therapy can be increased, and this is an opportunity to enhance therapeutic outcomes.
The complexity of inhalation products makes it more difficult to develop generics versions (substitutable and interchangeable). Under Article 10.3 hybrids or 505(b)(2), the development of orally inhaled products can be approved. Cost-effective versions like this have good marketing opportunities in emerging markets.
The development of orodispersible tablet technology
The development of orodispersible tablet technology is becoming more important because of increasing demand and the availability of technology that allows its manufacture using conventional equipment. This technology is more convenient for the patient and has the ability to increase market share owing to product differentiation [25].
Intranasal diazepam as an alternative to diastat rectal gel, developed by Neurelis
Intranasal diazepam (NRL-1) is a proprietary formulation of diazepam delivered via an already marketed nasal sprayer, that is being developed for the management of children and adults who require intermittent use of diazepam to control bouts of acute repetitive seizure activity. In clinical trials, NRL-1 has demonstrated high bioavailability, low variability from dose to dose, and was well tolerated. Most patients who experience acute repetitive seizures, however, are currently seen in emergency rooms and treated with intravenous benzodiazepines [26]. Most of these patients are admitted to hospital. Intranasal diazepam has the potential to provide a superior alternative to either rectal administration of Diastat® or the need to visit the emergency room for intravenous administration of drugs.
The acceptance barrier for the super generic has been raised considerably owing to pressure on health funds. For example, in the US, Express Scripts and CVS Health have recently removed two combination products [27] from their reimbursement list [28]. These are Duexis and Vimovo; both are combination drugs and their ingredients are available as generic individual monotherapies. Duexis comprises the pain reliever ibuprofen, the active ingredient in Advil and Motrin, and the heartburn remedy famotidine, Pepcid®. Vimovo is another pain drug and stomach combination drug, naproxen (Aleve) and esomeprazole (Nexium). These two products both belong to Horizon Pharma and the removal of such products will require companies looking for differentiation to refocus their targets. In the case of Vimovo, Horizon has advised that they have been awarded additional [29] patent claims, but anticipate that the removal of the product from reimbursement will reduce income and they will be looking at alternate means for promoting such products as speciality pharmaceuticals or even rising prices [30].
Discussion
In this paper, the ambiguities in designations of the super generics are discussed, and differences between them are explored through interviews and literature review. These new versions may have different periods of exclusivity in different jurisdictions, and that is one of the reasons that designation is so important [31, 32].
For the 505(b)(2) pathways commonly used by super generic producers, more products were approved in 2012 through 505(b)(2) development in the US [31]. This is because the super generic pathway can be applied to a wide range of development scenarios. Bringing modified version of an existing drug to market, although potentially much faster and less costly than starting a new drug, is still a demanding process, requiring a complete understanding of marketing challenges around product designation [31–33].
The super generic or value-added generic created a new product strategy and opened a new pathway towards sustainability in the market. The benefit of this product differentiation is not only focused on higher financial outcomes, albeit at higher risk for the companies, but also, most importantly, on providing a range of affordable alternatives to patients.
In this changing perspective for the generics industry, using the ‘generics’ designation for improved therapeutics seems to present an obstacle to generating higher revenues. Nowadays, the major generics manufacturers need to demonstrate their potential for going far beyond generics activities. The need for generics manufacturers to differentiate in the generic pharmaceuticals market is driven by the necessity to move away from the classic International Nonproprietary Name market with low margins and falling prices.
Even if switching to super generics seems to be ‘hard’, these differentiated versions are addressing unmet patient needs and may provide value to payers and justify a higher than generics price.
These pharmaceutical products occupy a space between innovative and generics, ‘the third sector’, and is forecasting now at US$24 billion and reaching US$32 billion global sales by 2018 [33].
Another important concern here is prioritizing patients’ needs as a ‘value’. This can contribute to progress in the context of ‘patient centric’ approach.
Super generic products are introduced to market as innovative or re-innovated pharmaceuticals. The other dimensions of super generics include a cost-saving paradigm [34], patient compliance providers, and pharmaceutical growth generators [35].
In spite of their high economic and therapeutic values, the use of super generics seems to have decreased and interest partly diminished. Even if the use of 505(b)(2) as a regulatory pathway in the US can provide market exclusivity, semantic challenges around the use of ‘generic’ drug for the product category can be the main drawback.
Conclusion
If innovative generics companies can invest in generics and re-innovated, improved therapeutics, they may take a big step towards new chemical entity development, and, in the long run, may benefit from the both types of products by value proposition. The semantic challenge can be observed as a strategic marketing for these products.
Acquiring higher reimbursement prices can become reality if, and only if, any agreed price increase matches the effectiveness of the value-added product. If it is innovative and reduces the risks, then payers may accept a higher price.
These improved versions offer other benefits. For example, encouraging payers for better alternatives; providing different affordable pharmaceutical treatments to patients; designing products that meet more precisely the needs of patients; and improving the standards of incremental product innovation and life-cycle strategy.
Value integration strategies, however, may also include educating prescribers to better choose their target treatments; and communicating with patients to inform them of alternatives that may be more convenient to administer, and with similar or improved clinical outcomes.
Acknowledgement
The author wishes to thank the English editing support provided by Ms Maysoon Delahunty, GaBI Journal Editor for this manuscript.
Disclosure of financial and competing interests: Dr Malcolm Ross is Managing Director of Generapharm Consulting, Basel, Switzerland. The authors have indicated that they have no conflicts of interest with regard to the content of this article.
Provenance and peer review: Not commissioned; externally peer reviewed.
Co-author
Malcolm Ross, BPharm, PhD, Switzerland
References
1. Phelps KV. Taking the 505(b)(2) route. Drug Discovery & Development. 9 August 2012 [homepage on the Internet]. 2015 Feb 24 [cited 2015 Feb 25]. Available from: http://www.dddmag.com/articles/2012/08/taking-505b2-route
2. Robinson D, Reed V, editors. The A Z of social research jargon. Aldershot, UK: Ashgate; 1998.
3. Kermani F. Generics companies: aspiring to innovate. Journal of Generic Medicines. 2004;1:4(336-46).
4. Sommerfeld TL. Generic appeal to innovation pharma. Journal of Generic Medicines. 2007;4:4(259-66).
5. Stopler W. Joseph Alois Schumpeter: the public life of a private man. Princeton: University Press; 1994.
6. Schumpeter J. The Theory of economic development. Harvard Economic Studies 46; 1934.
7. Wechsler J. New era for generic drugs. 1 November 2014. BioPharm International. [cited 2015 Feb 25]. Available from: http://www.biopharminternational.com/new-era-generic-drugs-2
8. Actavis. FDA Advisory Committee recommends against approval of Actavis’ Nebivolol/Valsartan fixed-dose combination NDA for treatment of hypertension. 9 Sep 2014 [homepage on the Internet]. [cited 2015 Feb 25]. Available from: http://www.actavis.com/news/news/thomson-reuters/fda-advisory-committee-recommends-against-approval
9. Barei F, Le Pen C, Simoens S. The generic pharmaceutical industry, moving beyond incremental innovation. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):13-9. doi:10.5639/gabij.2013.0201.011
10. New Pharma Thinkers. Barei F. The assessment of innovation in generic pharmaceutical industry, a qualitative review. 18 September 2012 [homepage on the Internet]. [cited 2015 Feb 25]. Available from: http://www.newpharmathinkers.com/super-generic-innovation/
11. Ross M. Innovation strategies for generic drug companies: moving into super generics. IDrugs. 2010;13(4):243-7.
12. Stegemann, et al. Improved therapeutic entities derived from known generics as an unexplored source of innovative drug products. Eur J Pharm Sci. 2011;44(4):447-54.
13. Stegemann, et al. Developing and advancing dry powder inhalation towards enhanced therapeutics. Eur J Pharm Sci. 2013;48(1-2):181-94.
14. Cheng C, Shiu E. Re-innovation – what is it and what could influence its performance? Academy of Marketing Conference; 2006: London, UK. Conference Paper No. 254.
15. Rothwell R, Gardiner P. Re-innovation and robust designs: producer and user benefits. Journal of Marketing Management. 1988;3(3):372-87.
16. Sainkoudje, SI. Analyse de la procédure 505(b)(2) de la réglementation américaine relative aux demandes d’autorisation de mise sur le marché de médicaments génériques innovants et comparaison avec les procédures similaires en Europe et en France. Université Rennes 1, Faculté de la pharmacie.
17. Teva Pharmaceuticals Industries Ltd [homepage on the Internet]. 2015 Feb 24 [cited 2015 Feb 25]. Available from: http://www.tevapharm.com/RandD/Documents/BioMed/RandD.pdf
18. U.S. Food and Drug Administration. Guidance for Industry. Applications covered by section 505(b)(2). Draft guidance. October 1999 [homepage on the Internet]. 1999 Oct 4 [cited 2015 Feb 25]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm079345.pdf
19. Daiichi Sankyo. Daiichi sankyo launches new generic drugs through its Daiichi Sankyo Espha subsidiary. 20 June 2014 [homepage on the Internet]. 2015 Feb 24 [cited 2015 Feb 25]. Available from: http://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006145.html
20. Porter ME, Kramer MR. Creating shared value. Harvard Business Review. 30 November 2011 [homepage on the Internet]. 2014 Apr 17 [cited 2015 Feb 25]. Available from: http://adamantconsult.com/wp-content/uploads/2014/05/11-porter-creating-shared-value-ss-highlights.pdf
21. Trivedi B, et al. Quality by design in pharmaceuticals. Int J Pharm Pharm Sci. 2012;4(1):17-29.
22. Cosby ST. Process analytical technology in biopharmaceuticals. Massachusetts Institute of Technology; 2013. 2013 May 9 [cited 2015 Feb 25]. Available from: http://dspace.mit.edu/bitstream/handle/1721.1/80911/857788914.pdf?sequence=1
23. Maggio ET. Absorption enhancing excipients in systemic nasal drug delivery. Journal of excipients and food chemicals. 2014;5(2). Available from: https://ojs.abo.fi/index.php/jefc/article/download/756/1030
24. Schoubben A, Blasi P, Marenzoni ML, Barberini L. Capreomycin supergenerics for pulmonary tuberculosis treatment: preparation, in vitro, and in vivo characterization. Eur J Pharm Biopharm. 2013;83(3):388-95.
25. Swapnil SG, et al. A review on: Orodispersible Tablet Technology – a novel approach to develop the supergenrics. International Journal of Pharmaceutical Science Research. 2014;26(2):231-6.
26. Neurelis regains rights to Intranasal Diazepam Program. [cited 2015 Feb 25]. Available from: from: http://www.lelezard.com/en/news-4322972.html
27. Controlled Release Society. CRS Newsletter [homepage on the Internet]. 2011;28(1). [cited 2015 Feb 25]. Available from: http://www.controlledreleasesociety.org/publications/Newsletter/Documents/v28i1.pdf
28. Langreth R. More medicines goes off limit in drug price showdown. Bloomberg Business. 25 November 2014. [cited 2015 Feb 25]. Available from: http://www.bloomberg.com/news/2014-11-25/more-medicine-goes-off-limits-in-drug-price-showdown.html
29. Complete response letter from FDA, positive opinion on drug, technical update, financial result release, and clinical trial results – research reports on Acelrx, Horizon, Gilead, Abbvie and Exelixis [press release]. Market Watch. 31 July 2014. [cited 2015 Feb 25]. Available from: http://www.marketwatch.com/story/complete-response-letter-from-fda-positive-opinion-on-drug-technical-update-financial-result-release-and-clinical-trial-results-research-reports-on-acelrx-horizon-gilead-abbvie-and-exelixis-2014-07-31
30. Helfand C. Doom for combo meds? Expresse scripts, CVs dump horizon pharma two-in-one drugs. Fierce Pharma Marketing. 30 July 2014. [cited 2015 Feb 25]. Available from: http://www.fiercepharmamarketing.com/story/doom-combo-meds-express-scripts-cvs-dump-horizon-pharma-two-one-
31. Phelps K. (B)(2) or not (B)(2) – That is the Question, 505(b)(2) can result in product approval with lower risk, reduced costs, and faster time to market? Contract Pharma. 6 November 2013. [cited 2015 Feb 25]. Available from: http://www.contractpharma.com/contents/view_experts-opinion/2013-11-06/b2-or-not-b2-that-is-the-question/
32. Szejtili J. Cyclodextrin complex generic drugs are generally not bio-equivalent with the reference products: therefore the increase in number of marketed drug/Cyclodextrin formulations is so slow. Journal of Inclusion Phenomena and Marocyclic Chemistry. 2005;52(1-2):1-11.
33. Vaczek D. Finding value in generics with a twist. Medical Marketing and Media. 5 November 2013. [cited 2015 Feb 25]. Available from: http://www.mmm-online.com/finding-value-in-generic-drugs-with-a-twist/article/381633/
34. Garud SS. (2014), Super generics a cost-saving paradigm. IJPRD. 2011;5(11):57-66.
35. Kumar L, Deshpande A, Page A. Super generics/improved therapeutic entities: an approach to fulfill unmet medical needs & extending market exclusivity of generic medicines. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(2).
|
Author for correspondence: Fereshteh Barei, PhD, Paris Dauphine University, Place de Maréchal de Lattre de Tassigny, FR-75016, Paris, France |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2015 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.