Biosimilars naming, label transparency and authority of choice – survey findings among European physicians
Published on 2014/03/24
Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(2):58-62.
|
Introduction: A survey of the views of European physicians on familiarity of biosimilar medicines has demonstrated the need for distinguishable non-proprietary names to be given to all biologicals. |
Submitted: 11 March 2014; Revised: 15 May 2014; Accepted: 19 May 2014; Published online first: 2 June 2014
Introduction
With the growing number of biosimilar medicines on the European market [1], the Alliance for Safe Biologic Medicines (ASBM) has completed a survey of European physicians to:
- examine attitudes of European physicians on biosimilar naming and substitution
- assess physician knowledge, sources of information and need for further education on biosimilars and
- provide data to put policy developments at EU and national level into perspective and inform policy recommendations.
Responses from 470 prescribers located in France, Germany, Italy, Spain and the UK were collected and analysed. Respondents were all specialists who prescribe biologicals, including nephrologists, rheumatologists, dermatologists, neurologists, endocrinologists and oncologists. The perspective of European physicians reflects hands-on clinical experience with biologicals in a therapeutic setting and highlights the point that non-proprietary names matter to patient safety.
The findings point to some confusion among physicians in Europe in the area of biological and biosimilar medicines, which indicates the need of further education [2] with proper information such as the Consensus Information Paper 2013 published by the European Commission [3]. Physicians were not in agreement on where the authority lies over selecting the most suitable biological medicine for a patient – with the physician and patient or with the pharmacist.
The absence of a Europe-wide agreement on how biological and biosimilar medicines are recorded was also identified. This will need to be rectified in order to achieve effective pharmacovigilance, limiting possible adverse events in the future [4].
Methods
By the last quarter of 2013, a total of 4,324 survey invitations were sent to prescribers in France, Germany, Italy, Spain and the UK. Participants were selected from a large global market research panel of prescribers; 1,002 responded, giving a total response rate of 23.1%. 62 of the 1,002 screened out. 470 prescribers (20% from each of the five European countries) completed the survey. Oncologists were paid the US equivalent of $32.00 to complete the survey. All other participants were paid the US equivalent of US$25.00. All surveys were presented in the local language (English, French, German, Italian and Spanish). Prescribers answered questions in a 15-minute web-based survey.
Prescribers included nephrologists (18%), rheumatologists (17%), dermatologists (17%), neurologists (16%), endocrinologists (16%) and oncologists (16%). They were based in hospitals (58%); academic medical centres (24%); private, family practices (8%); community settings (8%); multi-specialty clinics (2%); or other settings (1%).
Most physicians (46%) had 11–20 years’ experience, while 18% had 6–10 years, 28% had 21–30 years, 7% had more than 30 years and only 1% had 5 years’ or less experience. Nearly three quarters (70%) conducted more than 50 patient appointments a week, while a third (29%) conducted 20–50 appointments, and 1% conducted fewer than 20. Of these, the vast majority (92%) prescribed biological medicines. Three quarters of physicians (76%) said they knew that their patients were treated with biological medicines by other healthcare providers; while 12% knew that their patients were not treated with biological medicines elsewhere and the remaining 12% were not sure.
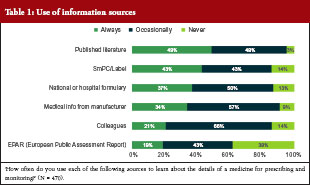
Only 19% of the surveyed physicians said they always used the European Public Assessment Report (EPAR) to learn about the details of a medicine for prescribing and monitoring, while 43% used it occasionally and a similar proportion (38%) never used it at all. Over 80% of physicians used the summary of product characteristics (SmPC) and the label to learn about the medicine either all of the time (43%) or some of the time (43%). Transparent information in the SmPC and the label are relevant for appropriate physician information. Other sources of information used are shown in Table 1.
Results
Prescriber’s knowledge of biosimilars
The prescribers’ overall knowledge of biologicals and biosimilars was ascertained on the basis of questions related to their understanding of these medicines coupled with information on where they had gained this understanding (at meetings, in journals or from biological or biosimilar companies, etc).
Most physicians (46%) responded that they had only a basic understanding of biological medicines, while 43% said they had a complete understanding. Only 1% of all physicians surveyed had never heard of biological medicines, while 11% were not able to define them. These results varied by country, a relatively high proportion of physicians surveyed from Spain (62%) were ‘very familiar’ with biological medicines, while this figure was only 30% in France. Results from the remaining countries stood at around 40%: Germany (39%), Italy (42%) and the UK (40%).
Over half (54%) of those surveyed reported that they are ‘familiar’, but with only a basic understanding, with biosimilar medicines, while 20% were unable to define biosimilars and 4% had never heard of them. Only 22% of physicians surveyed were ‘very familiar’ with a complete understanding of biosimilar medicines. As before, this varied by country; a higher proportion of physicians in both Germany (59%) and the UK (59%) were ‘familiar with a basic understanding’ with biosimilar medicines compared with physicians in France (44%).
The findings highlight an urgent need for further education of physicians and others related to the prescribing of these medicines. Further dialogue and collaboration between physicians, authorities and the healthcare biotech industry continues to be a priority. With this in mind, it is important to ascertain where physicians currently find out information about biosimilars.
The question of how physicians had become familiar with biosimilars was answered by only 357 of the 470 recruits (76%). Most of these gained familiarity through attending conferences and seminars (47%), while 35% learnt through self-study, 11% through studies sponsored by biosimilar companies and the remaining 6% split equally between studies sponsored by innovator companies, clinical trial participation and other routes. Prescribers in Spain were relatively more likely to have learnt about biosimilars through biosimilar company-sponsored study (21%) and relatively less likely to have learnt through self-study (20%). Self-study was more likely among physicians in France (37%), Germany (44%), Italy (31%) and the UK (45%), where the importance of scientific publishing is apparent. On this note, although only 20% of physicians in Spain learnt about biosimilars through self-study, 38% said they would prefer to learn through scientific publications. The preference for learning through scientific publications was shared by physicians in Germany (37%), Italy (44%) and Spain (39%).
Biosimilar approval awareness
Asked whether they were aware that a biosimilar might be approved for several or all indications of the innovator product on the basis of clinical trials in only one of these indications, over a third (37%) of all 470 physicians in the study believed that all indications have been clinically tested. The finding highlights a worrying lack of understanding in this area, making the case for further education and improved, more informative, and transparent labelling. There was a higher level of awareness of indication extrapolation among physicians in Italy, where 78% responded that biosimilars could indeed be approved for several or all indications of the innovator product on the basis of clinical trials in only one indication. 63% of prescribers in France responded that biosimilars could be approved for several or all indications, alongside 52% of physicians in Germany, 65% in Spain, and 55% in the UK.
Recording biologicals
Accurate recording is the linchpin of effective pharmacovigilance. In this section of the survey, physicians were asked how biological medicines were prescribed and recorded, and how adverse events were reported.
The vast majority (95%) of all physicians surveyed would identify any prescribed medicine, including biologicals, in the patient record, although this was slightly less likely in Germany (87%) and Spain (92%). If a patient was receiving a biological medicine prescribed by another healthcare provider, this was not identified in the patient record in 11% of cases. Again, this varied by country: a higher proportion of physicians from the UK (12%) and Germany (33%) do not record this information.
The question of how biological medicines were identified for prescription or in a patient record illuminated a worrying lack of Europe-wide convention. Based on answers from 417 of the physicians questioned, there was a three-way split in the way that biological medicines for prescription or recording in a patient record were identified between: (i) brand name and non-proprietary name (32%); (ii) brand name (30%); and (iii) non-proprietary/generic name (24%). A sizeable proportion (14%) answered that identification varies by medicine. The brand name was used most widely in France (53%) and Germany (40%), while brand name and non-proprietary name were more likely to be used in Italy (42%). The non-proprietary name/generic name was most likely to be used in the UK (37%).
Reporting adverse events
Alongside questions on how biological medicines were identified, physicians were asked how medicines were identified when reporting an adverse event (AE). Medicines were identified by both brand name and non-proprietary name by 54% of the 470 physicians questioned, by brand name by 29% of physicians, and by non-proprietary/generic name by 17% of physicians. Products were most likely to be recorded by brand name by physicians in France (58%) and Germany (36%).
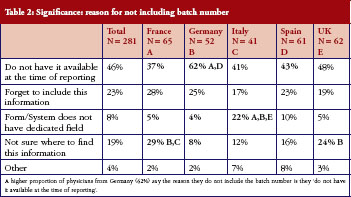
Batch number inclusion when reporting an AE varied widely: from always (40%); to sometimes (33%); to never (27%). Batch number was always included by 57% of physicians in Italy and 45% of physician in Germany, but never included by 38% of physicians in France and 43% of physicians in Spain.
Physicians who did not routinely include batch numbers when reporting AEs (answering ‘Sometimes’ or ‘Never‘ to this question) were questioned further, highlighting areas that will need to be addressed before this data, essential to successful pharmacovigilance, can be included routinely, see Table 2. Of 281 physicians (60% of all those questioned) who did not routinely include batch number, a relatively high proportion of physicians from Germany (62%) said they did not have the number available when they reported the AE. Nearly a third of physicians in France (29%) and a quarter of physicians in the UK (24%) did not know where to find the information.
Non-proprietary name implications
The fact that biosimilars, in contrast to generic drugs, can have different structures and therapeutic profiles, and can be approved for less than all indications of the reference product, may be lost if two distinct medicines have the same non-proprietary scientific name. Clearly, it is potentially unsafe to assume these products are identical and approved for the same indications.
Answers to the question ‘If two medicines have the same non-proprietary scientific name, does this suggest to you or imply that the medicines are structurally identical’ highlight considerable confusion among physicians. Over half (53%) of physicians questioned held the incorrect assumption that these products are structurally identical. A surprising 15% had no opinion either way, which could suggest that the respondents did not understand the question. Among those more likely to believe that these products are structurally identical were physicians from France (59%), Germany (68%) and the UK (59%).
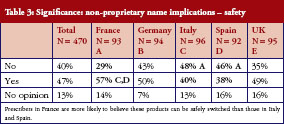
In turn, this leads to confusion over whether different medicines with the same non-proprietary name can be safely switched (between treatments or during a treatment course) or substituted. Asked whether two medicines with the same non-proprietary scientific name could be given safely to a patient with the same result, 40% said no, 47% said yes, and the remaining 13% had no opinion. There is no clear pattern here, prescribers in France (57%) were slightly more likely to believe their products could be safely switched than those in Italy (40%) and Spain (38%), but there were sizeable proportions of ‘yes’ and ‘no’ answers in every country surveyed, see Table 3.
The complexity is further illustrated by answers to the question that ‘if two medicines have the same non-proprietary scientific name, does this suggest to you or imply that a patient could be safely switched between the products during a course of treatment and expect the same result as treatment with only one of the products’. Thirty-nine per cent of physicians believed patients could be safely and effectively switched during a course of treatment, 45% believed they could not, and 16% had no opinion. Nearly half (49%) of prescribers in France believed switching was safe, while only 34% of physicians in Spain believed this.
Similarly, the physicians questioned were unclear whether two medicines with the same non-proprietary scientific name are approved for the same indications: 61% said they were; 31% said they were not; and 9% had no opinion. In other words – and these findings did not vary between countries – two thirds of prescribers do not have an understanding of the complexity and sensitivities surrounding biosimilars.
Clear naming and labelling is paramount. If a patient has an adverse reaction, which can occur months after receiving a biological medicine, the medicine needs to have been properly identified from the start. A clear naming system is essential in order to make identification possible, thereby enhancing access to these life-changing therapies, while also protecting patient safety.
Pharmacy substitution
The question of authority over selecting the most suitable biological medicine for a patient was posed in this survey, and physicians’ responses revealed a range of opinions across Europe. Of 470 physicians questioned, 24% thought it was critically important to have the sole authority to decide, together with their patients, the most suitable biological medicine for their disease. 48% thought it was ‘very important’, 23% thought it was ‘somewhat important’, 4% thought it was ‘slightly important’ and 1% thought it was ‘not important’.
These responses varied by country, with the highest proportion of physicians in Italy (34%) and Spain (33%) considering sole authority ‘critical’. Sole authority was considered only ‘very important’ or ‘somewhat important’ by physicians in Germany (38% and 37% respectively).
The importance of ‘dispense as written’ (DAW) or ‘do not substitute’ showed a similar pattern. Overall, 27% considered it critical, 47% thought it was ‘very important’, 20% thought it was ‘somewhat important’, 5% thought it was ‘slightly important’ and 1% thought it was ‘not important’. In Spain, 41% of physicians considered DAW critical, while only 13% of physicians in Germany considered it critical.
Most physicians among the 470 questioned felt it was important that pharmacists provide notification that their patient had received a biological other than the one prescribed if the patient was receiving chronic (repeated) treatment: 30% considered notification critical, 47% considered it very important, 16% somewhat important, 6% slightly important and 1% considered it not important.
Similarly, 62% of all physicians considered it not acceptable if the pharmacist decided which biological (innovator or biosimilar) to dispense, 35% considered it acceptable and 3% considered it totally acceptable. Unilateral decision making at the pharmacy was not considered acceptable by most prescribers, particularly in Italy where 77% of physicians considered it unacceptable.
Asked to define ‘bio-naïve’, 76% of physicians in the survey believed that this meant ‘a patient who has never received any biological treatment of this class.’ This was equally accepted across the countries studied, although slightly less likely in Germany, where only 66% of physicians agreed with this definition, see Table 4.
Conclusion
Biological medicines have had a profound effect in many medical fields, from oncology to neurology and across a host of other debilitating diseases, but the complexity of the molecule and its manufacturing process result in significantly higher cost than that of the small-molecule medicine. As the patents on biologicals begin to expire, it is hoped that the arrival of biosimilars – drugs that are similar, but not identical, to these innovator biologicals – will reduce the financial burden on healthcare systems [5]. But to benefit from these medicines it is crucial that prescribing physicians understand what these medicines are, and what these medicines are not.
The responses of European physicians recorded in this study reflect serious gaps in what is known about biological drugs in general and biosimilars in particular. There are several misconceptions regarding biologicals, and considerable education is needed in the area of differences between generic products and biosimilar products.
Unlike generic drugs, biosimilars are not identical to the innovator biological on which they are based; they have different structures, may have a different therapeutic profile, and may not be approved for all the indications for which the reference product was approved.
There is a clear need for distinguishable non-proprietary names to be given to all biological medicines to ensure intended prescribing as well as to support product identification when reporting and tracing adverse events [6–8].
Increasing numbers of biological medicines, both originator and biosimilar, are being approved around the world. According to the findings in this study, most physicians use the SmPC and the label to learn about a medicine, illustrating how important it is that clear information, and informative labelling, is provided for every biological and biosimilar.
With the co-existence of different biosimilars from different manufacturers, an effective pharmacovigilance system is urgently needed in order to allow accurate adverse events reporting. How these products are named will clearly play a central role in facilitating pharmacovigilance worldwide, supporting the safe use of these medicines [4, 8].
Alongside the prescribing physician, the pharmacist also plays a key role in effective pharmacovigilance. Switching between brand name pharmaceutical drugs and their generics presents little or no threat to patient safety since they should be identical, but switching between a biological drug and its biosimilar, or between different biosimilars, is not the same. Across Europe, this study revealed a range of opinions among prescribing physicians as to where the authority should lie when deciding on the most appropriate biological or biosimilar, with most insisting that physicians and their patients – not pharmacists – should have sole authority when making these decisions. It is important for most physicians to retain the authority to use ‘do not substitute’ to ensure the patient receives the correct medicine.
In addition to a clear need for further education for physicians who prescribe these medicines, distinguishable non-proprietary names are important to practising physicians, and we hope this system will be used by the World Health Organization in crafting a global standard that will improve patient safety worldwide [1].
The findings of this study echo, and reinforce, concerns already raised by the medical community. Effective Europe-wide, and by extrapolation worldwide, pharmacovigilance is urgently needed in order to benefit fully from the considerable advances offered by biological drugs and biosimilars, while limiting future adverse events.
|
Key points of the 2014 European prescribers’ survey
|
Acknowledgement
The authors wish to thank the medical writing and editorial support by Dr Bea Perks, GaBI Journal Editor.
Funding sources
The Alliance for Safe Biologic Medicines (ASBM) is an organization composed of diverse healthcare groups and individuals – from patients to physicians, innovative medical biotechnology companies and others who are working together to ensure patient safety is at the forefront of the biosimilars policy discussion. The activities of ASBM are funded by its member partners who contribute to ASBM’s activities. Visit www.SafeBiologics.org for more information.
Disclosure of financial and competing interests: Dr Richard O Dolinar, Chairman of the Alliance for Safe Biologic Medicines (ASBM), and Mr Michael S Reilly, Executive Director; are employed by ASBM.
This paper is funded by ASBM and represents the policies of the organization.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Richard O Dolinar, MD, Chairman
Michael S Reilly, Executive Director
Alliance for Safe Biologic Medicines, PO Box 3691, Arlington, VA 22203, USA
References
1. Rovira J, et al. Biosimilars in the European market. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):30-5. doi: 10.5639/gabij.2013.0201.012
2. The future of biological therapy: a pathway forward for biosimilars Generics and Biosimilars Initiative Journal, 2013;2(1):36-40. doi: 10.5639/gabij.2013.0201.014
3. GaBI Online – Generics and Biosimilars Initiative. Biosimilars: what physicians need to know [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 May 15]. Available from: www.gabionline.net/Reports/Biosimilars-what-physicians-need-to-know
4. Clayton J. Tighter EU rules on pharmacovigilance for biologicals. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):56-7. doi:10.5639/gabij.2012.0102.015
5. Haustein, et al. Saving money in the European healthcare systems with biosimilars. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4). 120-6. doi:10.5639/gabij.2012.0103-4.036
6. Thorpe R, Wadhwa M. Terminology for biosimilars–a confusing minefield, Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):132-4. doi:10.5639/gabij.2012.0103-4.023
7. Alexander E. The biosimilar name debate: what’s at stake for public health, Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(1):10-2. doi:10.5639/gabij.2014.0301.005
8. Dolinar R. WHO leadership in public safety on biosimilars to be commendedGenerics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(4):167. doi:10.5639/gabij.2013.0204.047
| Author for correspondence: Michael S Reilly, Executive Director, Alliance for Safe Biologic Medicines, PO Box 3691, Arlington, VA 22203, USA |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2014 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.




Full report
This is nothing but motivated article to stop the biosimilars entering market after patent expiry.
The reply of the physicians are in the context of the questions being asked. The sponsoring body’s motivation is well understood and I think GaBI should be careful in giving publicity to such motivated articles.
Dear Dr Shah,
Thank you for sharing your comments. GaBI Journal manuscripts are peer reviewed by several expert peer reviewers presenting independent unbiased scientific information. Manuscripts of high value content are published. We would be delighted if you would consider submitting a Letter to the Editor to GaBI Journal to share your insight and knowledge on the subject. If interested, please feel free to reach us at editorial@gabi-journal.net. Thank you again and we hope to hear from you soon.
This is a very informative article descriping the very important survey covering most related factors to the safety of biosimilars
I would be very grateful if you can provide me with the questionnaire so as to benefit from such research in my community.
Dear GAUFRIAU,
We very much appreciate your kind feedback. The full report available on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office