Volume 14
2025
Issue 2
Highlights in this issue:
- Therapeutic equivalence of a formulation of purified micronized flavonoid fraction of diosmin/hesperidin 450 mg/50 mg in healthy adults: an open-label, randomized, single-dose, crossover study
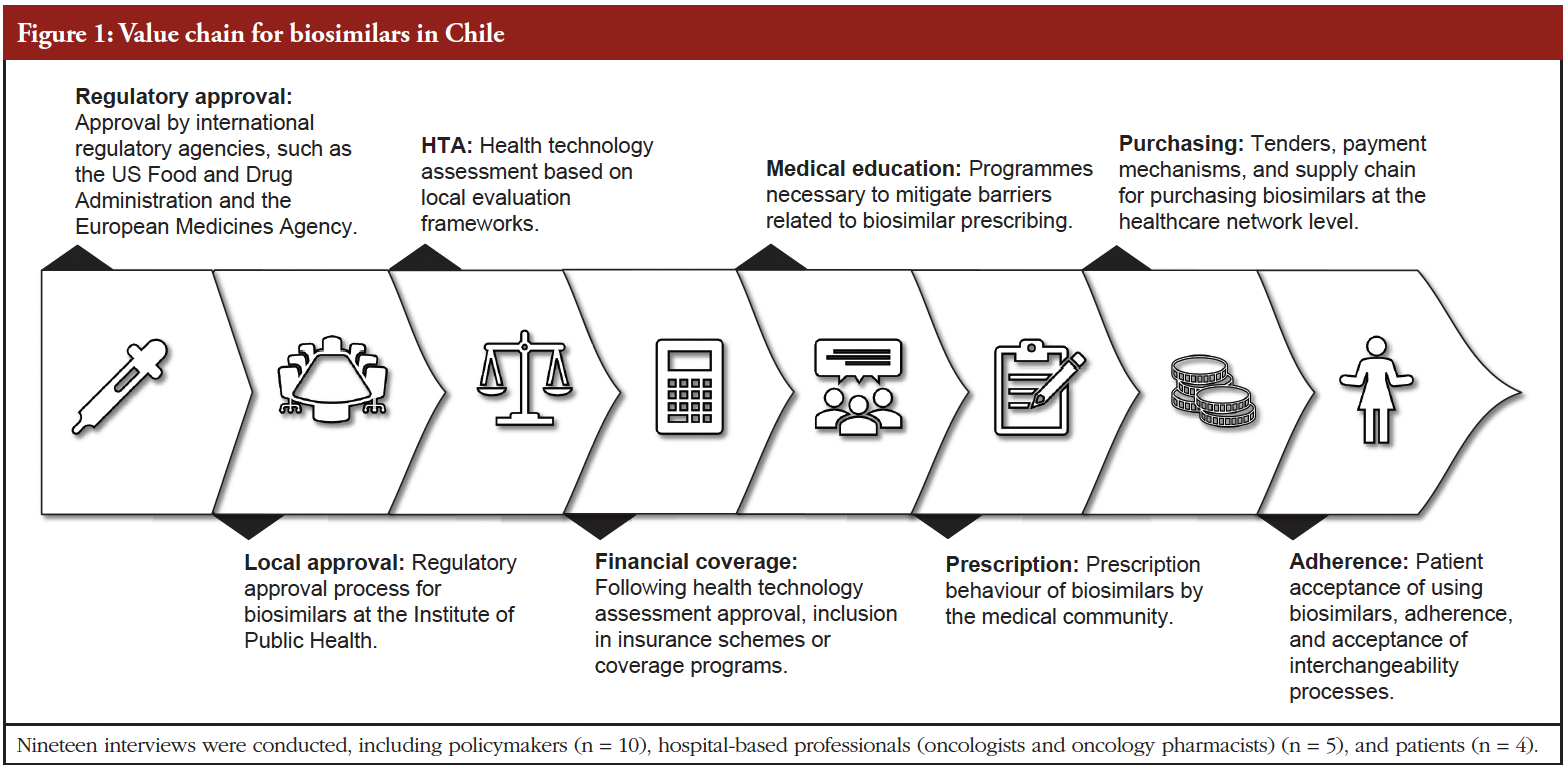
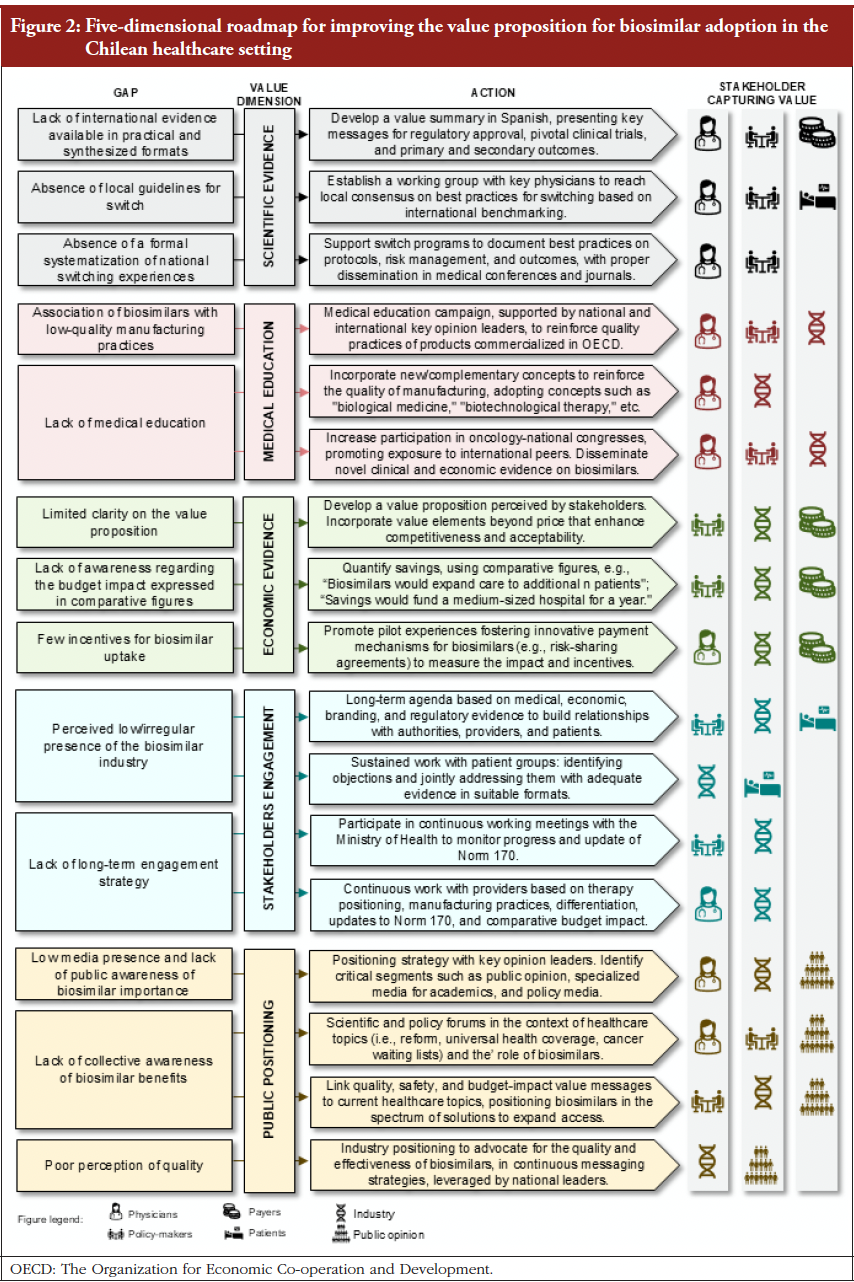
- Follow-on biologicals/biosimilars approved in Brazil: September 2025 update
- Current trends in biosimilar uptake and special focus on automatic substitution – a symposium report
126 views
Special Report – Stability Studies
The extended stability studies of anticancer therapies published in 2024 cover 21 small molecules across 10 parenteral drug groups in oncology.
Submit your Research
Practitioners in the scientific research area on the development and use of generics and biosimilar medicines who would like to author papers on related topics, and whose manuscripts comply with the editorial policy of GaBI Journal, are welcome to submit their manuscripts for assessment, peer review and publication in GaBI Journal.
For Authors
All articles are published electronically ahead of print with the definite citation line. Thus, an article will be available online shortly after the author’s approval to print.
Peer Review
All manuscripts submitted to GaBI Journal are subject to a rigorous peer review process.
We welcome suggestions for referees from the author though these recommendations may or may not be used. Names, postal and email addresses of three to four experts in the appropriate area of research should accompany each manuscript submission.
Article Processing Charges
The article will be published online after receipt of the corrected proofs. This is the official first publication citable with the DOI. After release of the printed version, the article can also be cited by issue and page numbers.
About GaBI
Sustainability and equal access to health care for all is at risk due to rapidly increasing costs. The reasons for this are diverse: increased standard of living and life expectancy, but also the high cost of new drug innovations. Today’s society is looking for inexpensive solutions to its ever-increasing demands. Healthcare systems endeavour on the one hand to provide groundbreaking, novel solutions to a greater than ever demand for therapeutic assistance, and on the other hand to achieve this in the most cost-efficient way.
Articles
Volumes






Subscribe
Sign up today for the weekly briefing on the latest developments in generic and biosimilar medicines!