Building stakeholder confidence in biosimilar medicines through evidence-based information sharing
Published on 2017/11/13
Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(4):181-2.
|
Abstract: |
Submitted: 23 October 2017; Revised: 6 November 2017; Accepted: 7 November 2017; Published online first: 20 November 2017
Introduction
As both clinical experience and real-world evidence increases, knowledge sharing and identifying best practices can support the appropriate use of biologicals, including biosimilar medicines. Gianluca Trifirò, Assistant Professor of Pharmacology at the University of Messina and a Clinical Pharmacologist in the Unit of Clinical Pharmacology of the Academic Hospital ‘G. Martino’ of Messina, Italy provided an update of the latest available clinical experiences with biosimilar medicines in Italy, focusing on switching between biological medicines and interchangeability. He provided clarity on the European Crohn’s Colitis Organisation (ECCO) position on the use of biosimilars for inflammatory bowel disease (IBD) and discussed the challenges ahead [1, 2].
EC stakeholder event
The European Commission (EC) held a stakeholder event to discuss biosimilar medicinal products in May 2017. A session on building stakeholder confidence in biosimilar medicines at this event provided an update of the latest available clinical experiences with biosimilar medicines, focusing on switching between biological medicines and interchangeability.
Professor Trifirò discussed the issue of stakeholder confidence from the perspective of physicians in Italy. He reported the results of a survey of 816 Italian physicians including their experience with regional or national drug policies and how this has affected their prescribing behaviour.
In addition, Professor Trifirò presented the conclusions of an updated position statement from ECCO on the use of biosimilars for IBD. He concluded his presentation by looking at the future challenges facing biosimilar uptake in Italy and worldwide and how these challenges should be met.
Italian physician survey
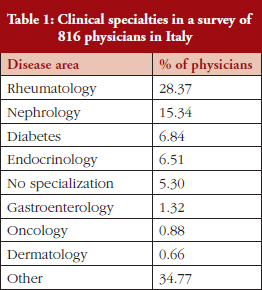
Professor Trifirò presented the results of a survey involving 816 physicians in Italy [3] and which was coordinated by Cittadinanzattiva that is a non-profit organization which is aimed at promoting civic participation and protection of citizens’ rights in Italy. The survey questionnaire was developed in a roundtable with representatives of several Italian scientific societies and patient and physicians organizations. The same roundtable participants distributed the survey questionnaire electronically using the web platform ‘esurveypro’ through a dedicated link through mailing, newsletter, Internet and participation at events and conventions.
The physicians, who worked across a range of specialties, see Table 1, were asked whether the way that they prescribe medicines was influenced by regional or national drug policies.
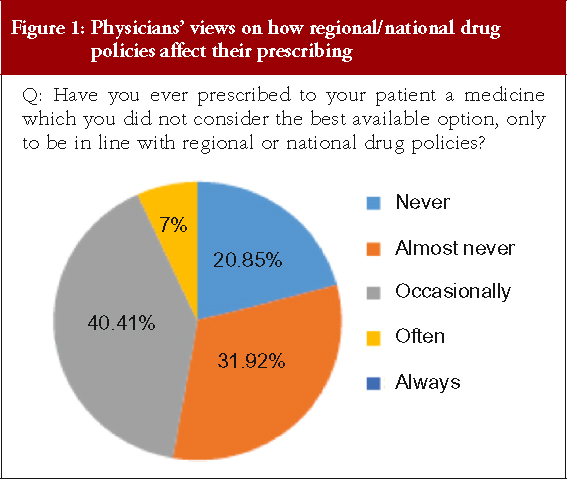
Physicians were asked whether they had ever prescribed a medicine that they did not consider was the best available option in order to conform to regional or national drug policies, see Figure 1, there was no response for ‘Always’.
ECCO position statement on the use of biosimilars for IBD
Professor Trifirò presented a position statement on the use of biosimilars for IBD released in 2017 by ECCO [1, 2]. The statement is the result of a consensus meeting held on 15 October 2016 and is based on the current regulatory guidance from the European Medicines Agency and evidence about efficacy and safety of biosimilars in IBD patients, see Box 1.
|
Box 1: ECCO 2017 position statement on the use of biosimilars for inflammatory bowel (IBD) disease [2] 1. Biosimilarity is more sensitively characterized by performing suitable in vitro assays than clinical studies. 2. Clinical studies of equivalence in the most sensitive indication can provide the basis for extrapolation. Therefore, data for the usage of biosimilars in IBD can be extrapolated from another sensitive indication. 3. When a biosimilar product is registered in the European Union, it is considered to be as efficacious as the reference product when used in accordance with the information provided in the Summary of Product Characteristics. 4. Demonstration of safety of biosimilars requires large observational studies with long-term follow-up in IBD patients. This should be supplemented by registries supported by all involved stakeholders [manufacturer, healthcare professionals and patients’ associations]. 5. Adverse events and loss of response due to immunogenicity to a biological drug cannot be expected to be overcome with a biosimilar of the same molecule. 6. As for all biologicals, traceability should be based on a robust pharmacovigilance system and the manufacturing risk management plan. 7. Switching from the originator to a biosimilar in patients with IBD is acceptable. Studies of switching can provide valuable evidence for safety and efficacy. Scientific and clinical evidence is lacking regarding reverse switching, multiple switching and cross-switching among biosimilars in IBD patients. 8. Switching from originator to a biosimilar should be performed following appropriate discussion between physicians, nurses, pharmacists and patients, and according to national recommendation. The IBD nurse can play a key role in communicating the importance and equivalence of biosimilar therapy. |
Future challenges
Several challenges for the future of bio-similars were identified. Establishing an effective system of pharmacovigilance will be key. The first challenge highlighted by Professor Trifirò will be to explore comparative long-term safety and effectiveness of first generation biosimilars thanks to data that have been cumulated over time. As experience with biosimilars grows, it will be important to evaluate clinical effects of switch between originator and biosimilars and vice versa, and between different originators.
Alongside these challenges, the secondary use of healthcare databases for post-marketing surveillance needs to be considered also for second-generation biosimilars in cancer patients.
While promoting the use of low cost biologicals, warned Professor Trifirò, prescribing biologicals wisely remains the highest priority over cost considerations. Every stakeholder (payers, healthcare professionals, patients) needs to be involved in the collection of real-world evidence about biosimilars that has to be integrated with pre-marketing evidence from randomized clinical trials [4].
Professor Trifirò concluded by noting that, given the growing number of biosimilars to be marketed in the near future across several different therapeutic areas, an international post-marketing surveillance system specifically for biosimilars must be established [5].
Acknowledgements
The author wishes to thank Dr Bea Perks, GaBI Journal Editor, in preparing this Special Report manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; internally peer reviewed.
Author
Assistant Professor Gianluca Trifirò1,2, MD, PhD
1Unit of Clinical Pharmacology, Azienda Ospedaliera Universitaria, Farmacologo Clinico, Policlinico ‘G Martino’, Dipartimento di Medicina, Clinica e Sperimentale, 1 Via Consolare Valeria, IT-98125 Messina, Italy
2Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, 1 Via Consolare Valeria, IT-98125 Messina, Italy
References
1. Danese S, Fiorino G, Raine T, Ferrante M, Kemp K, Kierkus J, Lakatos PL, Mantzaris G, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis. 2017;11(1):26-34.
2. GaBI Online – Generics and Biosimilars Initiative. ECCO position statement on biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 6]. Available from: www.gabionline.net/Biosimilars/General/ECCO-position-statement-on-biosimilars
3. Cittadinanzattiva. Squillace A, Amoroso C, Nardi S, Aceti T. Indagine civica sull’esperienza dei medici in tema di aderenza alle terapie, con focus su farmaci biologici e biosimilari [homepage on the Internet]. [cited 2017 Nov 6]. Available from: http://www.cittadinanzattiva.it/files/rapporti/salute/indagine-aderenza-terapie-focus-farmaci-biologici-biosimilari.pdf
4. Trifirò G, Ingrasciotta Y, Marcianò I, Genazzani A. Biosimilars in Italy: what do real-world data reveal? Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(3):114-9. doi:10.5639/gabij.2017.0603.023
5. van Gelder T, Lunddahl B, OM Claus B. Pharmacovigilance, traceability and building trust in biosimilar medicines. Generics and Biosimilars Initiative Journal (GaBI Journal). 2017;6(3):135-40. doi:10.5639/gabij.2017.0603.026
|
Author: Assistant Professor Gianluca Trifirò, MD, PhD, Unit of Clinical Pharmacology, Azienda Ospedaliera Universitaria, Farmacologo Clinico, Policlinico ‘G Martino’, Dipartimento di Medicina, Clinica e Sperimentale, 1 Via Consolare Valeria, IT-98125 Messina, Italy |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2018 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.