Follow-on biologicals/biosimilars approved in Brazil: May 2023 update
Published on 2023/03/02
Generics and Biosimilars Initiative Journal (GaBI Journal). 2023;12(2):67-72.
|
Abstract: |
Submitted: 29 September 2022; Revised: 26 January 2023; Accepted: 27 January 2023; Published online first: 9 February 2023
In Brazil, a legal framework for approving follow-on biological products using a comparability pathway was established in 2010, via the Resolution of the Collegiate Board of Directors (RDC) biosimilars regulations, RDC 55/2010 [1, 2], by the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA).
The Brazilian regulations (RDC 55/2010) are based on different regulations and guidelines from around the world, including the World Health Organization (WHO) Similar Biological Product Guidelines. The regulations follow the same scientific principles as the WHO guidelines but contain some differences due to the specific needs of Brazil [2].
This Resolution aims to establish the minimum requirements for the registration of new biological products and biological products in the country, aiming to guarantee the quality, safety and efficacy of these drugs. ANVISA does not have an approval pathway specific for biosimilars, and the Resolution provides two regulatory pathways; 1) comparability (follow-on biological) – a stepwise approach and 2) a stand-alone pathway (non-biosimilar), for non-originator biologicals [3].
ANVISA uses the terms of: (a) new biological product, for a new biological entity not yet registered; and (b) biological product, referring to the copy or follow-on products containing an active substance already registered [4].
In November 2022, ANVISA issued three new guidelines to regulate biologicals/biosimilars and radiopharmaceuticals [5].
Celltrion’s Remsima (infliximab) was the first biosimilar product approved in Brazil in 2015, by the comparative pathway and extrapolated a monoclonal antibody (mAb) indication for clinical indications outside those included in biopharmaceutical phase III studies (rheumatoid arthritis and ankylosing spondylitis).
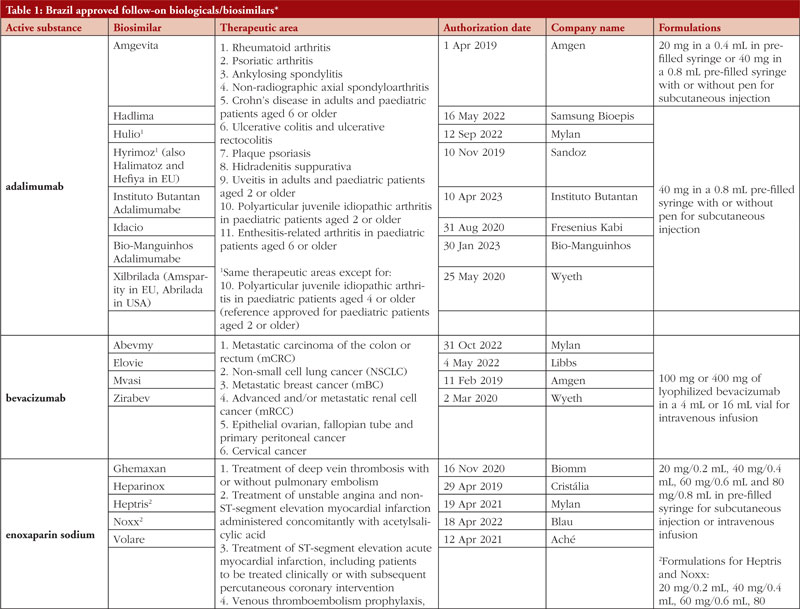
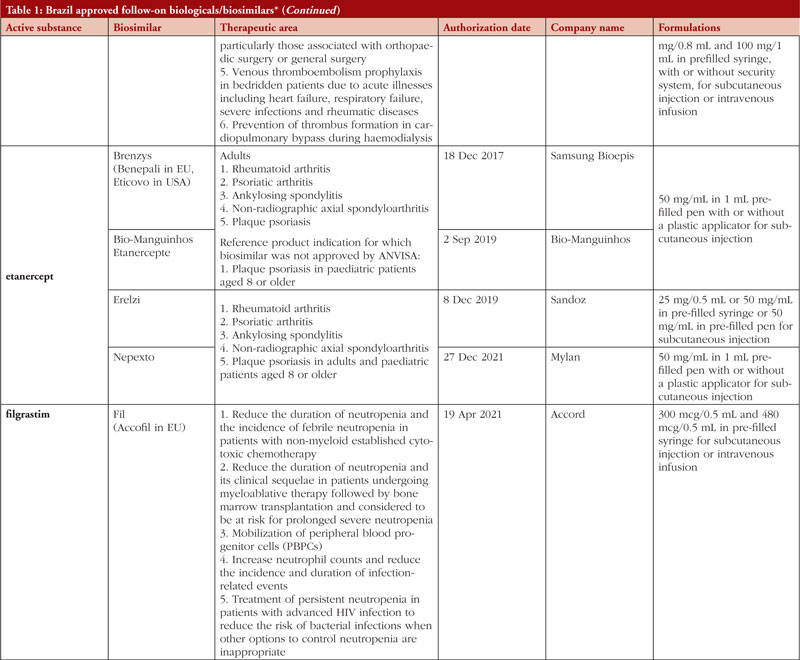
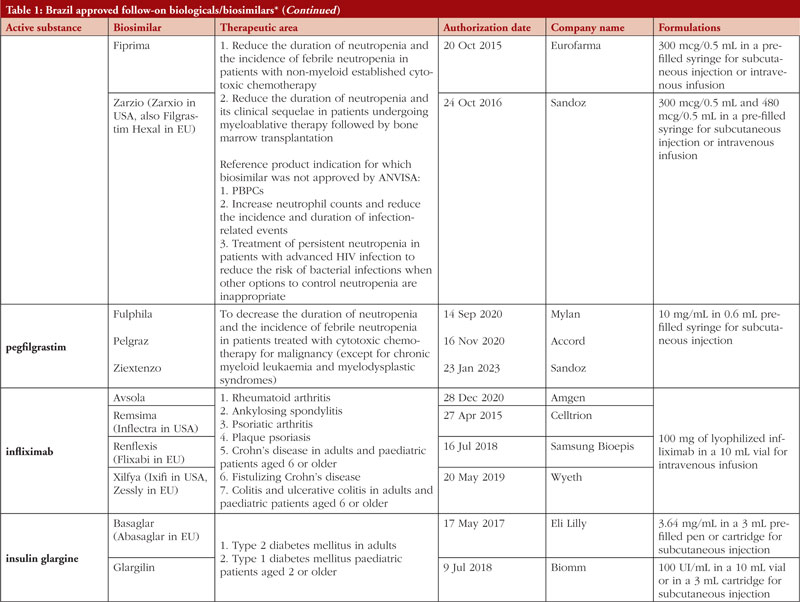
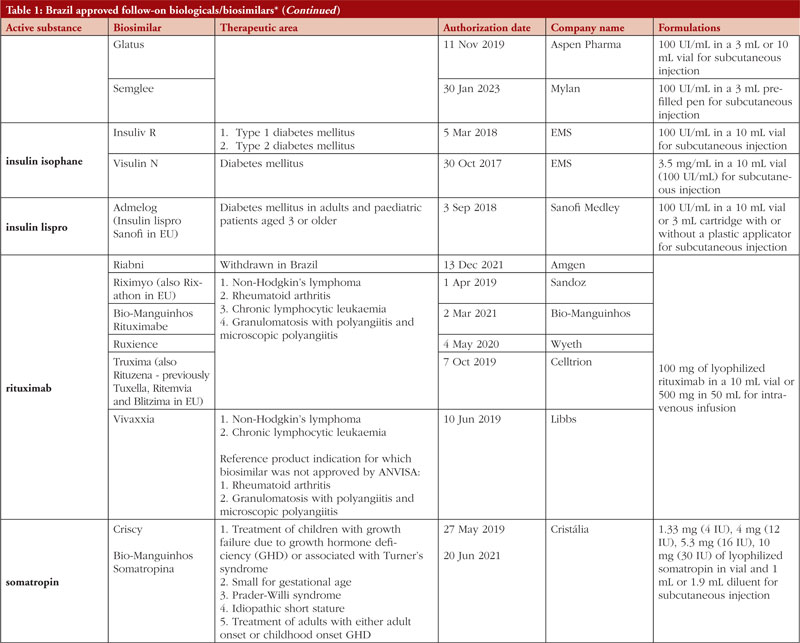
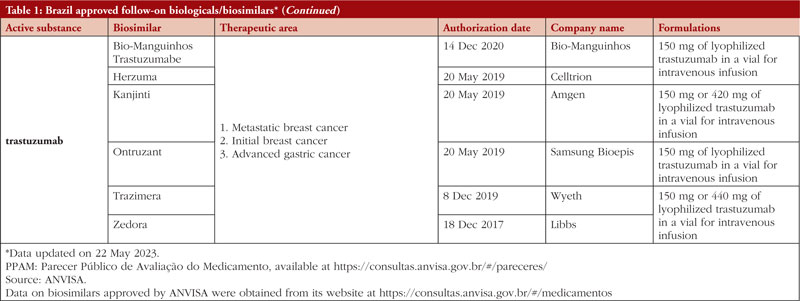
By middle of May 2023, ANVISA has approved 52 follow-on biological/biosimilar products that act by replacing naturally produced insulin, promoting growth, encouraging the bone marrow to produce more white blood cells, blocking tumour necrosis factor-alpha (TNF-α) or vascular endothelial growth factor (VEGF), causing the death of B-lymphocytes, inhibiting the proliferation of human tumour cells that overexpress human epidermal growth factor receptor 2 (HER2), and acting as an anti-thrombotic, see Table 1.
Most follow-on biologicals/biosimilars approved in Brazil have all indications of the reference product. Brazilian regulations allow for extrapolation if comparability in terms of safety and efficacy between products is demonstrated, if the mechanism of action and receptors involved for the different indications are the same, and if the safety and immunogenicity profiles of the biological product are sufficiently characterized. In addition, the dosage form and route of administration of the follow-on biological/biosimilar product must be the same as the reference product.
The timeline in the Brazilian regulatory pathway is known to be newer when compared to other regulatory authorities. approval processes for follow-on biologicals/biosimilars in Brazil are following an evolutionary route. Brazilian requirements are much more aligned with a strict regulatory process and ensure the same high standards of quality, safety and efficacy for follow-on biologicals/biosimilars as for originator biologicals.
Biosimilars have the potential to provide savings and efficiencies for healthcare systems, which can free up resources for other important aspects of health care, changing the sad reality of lack of access to high-cost medications in low- and middle-income countries and opening a new era of broadening access [4–9]. Adopting a transparent approach in educating different stakeholders and including them in shaping guidelines could contribute to the acceptance of follow-on biologicals/biosimilars in Brazil as has occurred in Europe [10].
Editor’s comment
ANVISA does not distinguish between real biosimilars, follow-on products which are not real biosimilars, and innovator products. The products listed in Table 1 could be any of these.
Off-patent biologicals approved prior to the implementation of RDC 55/2010 do not meet the criteria for classification as biosimilars, as specified in the regulations outlined in RDC 55/2010.
This manuscript’s list of off-patent biologicals/biosimilars does not include medications that received approval before the implementation of RDC 55/2010.
The medicines listed in this manuscript, approved as biosimilars, followed the regulatory process outlined in RDC 55/2010 and complied with the WHO guidelines.
Considering the global public health emergency caused by Sars-Cov-2, ANVISA published RDC 348/2020, which establishes extraordinary and temporary criteria and procedures for the registration and post-registration handling of medicines and biological products. This regulation allows for the inclusion of additional data and evidence to be submitted after registration through a Commitment. However, applicants must still adhere to the specific rules for registering their regulatory category, as well as any applicable supplementary regulations.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
1. Ministério da Saúde. Resolução de Diretoria Colegiada RDC 55/2010, Agência Nacional de Vigilância Sanitária (Brasília, DF), Dec. 16, 2010 [homepage on the Internet]. [cited 2023 Jan 26]. Available from: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2010/res0055_16_12_2010.html
2. GaBI Online – Generics and Biosimilars Initiative. Regulatory pathways for approval of biological products in Brazil [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jan 26]. Available from: www.gabionline.net/reports/Regulatory-pathways-for-approval-of-biological-products-in-Brazil
3. GaBI Online – Generics and Biosimilars Initiative. Key facts of biosimilars approval regulation in Brazil [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jan 26]. Available from: www.gabionline.net/biosimilars/general/key-facts-of-biosimilars-approval-regulation-in-brazil
4. GaBI Online – Generics and Biosimilars Initiative. Nomenclature of biologicals and biosimilars in Brazil [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jan 26]. Available from: www.gabionline.net/policies-legislation/nomenclature-of-biologicals-and-biosimilars-in-brazil
5. GaBI Online – Generics and Biosimilars Initiative. Guidelines for the regulation of biologicals, biosimilars and radiopharmaceuticals in Brazil [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jan 26]. Available from: www.gabionline.net/guidelines/guidelines-for-the-regulation-of-biologicals-biosimilars-and-radiopharmaceuticals-in-brazil
6. Pichon-Riviere A, Garay OU, Augustovski F, Vallejos C, Huayanay L, Del Pilar Navia Bueno PN, et al. Implications of global pricing policies on access to innovative drugs: the case of trastuzumab in seven Latin American countries. Int J Technol Assess Health Care. 2015;31(1-2):2-11.
7. Jackisch C, Lammers P, Jacobs I. Evolving landscape of human epidermal growth factor receptor 2-positive breast cancer treatment and the future of biosimilars. Breast. 2017;32:199-221.
8. Rehman K, Bukhari NI, Babar Z-U-D. Equitable access to biosimilars: an overview. In: Z-U-D. Babar, editor. Equitable access to high-cost pharmaceuticals. 1st ed. London, UK: Academic Press; 2018. p. 129-142.
9. Kim H, Alten R, Avedano L, Dignass A, Gomollón F, Greveson K, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020;80(2):99-113.
10. Cestari de Oliveira SH, Castanheira Alegria M, Stephano MA. Brazilian regulation of biosimilar products: what is important to know. BioPharm Int. 2022;35(9):30-37.
|
Author: Sílvia Helena Cestari de Oliveira, MSc. Pesquisadora de Biotecnologia Senior – Divisão de Biotecnologia, Cristália Produtos Químicos Farmacêuticos Ltda, Highway Itapira-Lindóia, Km 14 – Ponte Preta, CEP: 13970-970 Itapira, SP, Brazil |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2023 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.