Life begins at forty – hybridomas: ageing technology holds promise for future drug discoveries
Published on 2015/11/20
Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(1):21-6.
|
Abstract: |
Submitted: 26 October 2015; Revised: 8 January 2016; Accepted: 8 January 2016; Published online first: 22 January 2016
Hybridomas: ageing technology holds promise for future drug discoveries
American philosopher Walter B Pitkin said in 1932, ‘Life begins at 40’. The hybridoma platform was developed in 1975 as a means of antibody production, but it has not been until 2015 that their full potential may be realized. Hybridomas enabled the creation of mAbs and launched a multi-billion dollar industry. This technology is based on the fusion of a B lymphocyte cell and a tumour cell. The result of this fusion is known as a hybridoma, an immortal cell that produces specific antibodies. Though other platforms have since been developed, hybridoma technology enabled 40 years of mAb research. Newly devised methods of upstream and downstream processes, such as hybridoma selection, cell fusion and fusion partners, may lead to resurgence in the usage of the hybridoma platform. Here we review the history of hybridoma development, past and present usages of hybridomas, cell fusion techniques, antibody therapy, and trends in hybridoma technology.
History: Cesar Milstein, Father of the Hybridoma
Cesar Milstein wrote in his 1999 essay entitled, ‘The hybridoma revolution: an offshoot of basic research,’ that the term ‘hybridoma’ was coined in his lab in 1975 as a joke. A 1975 Nature article, ‘Continuous cultures of fused cells secreting antibody of predefined specificity’, is the earliest known reference to using hybridoma technology [1].
The first use of the term ‘hybridoma’ in PubMed searchable documents is from a 1979 paper, ‘Specific suppressor T cell hybridomas [2]’. Cesar Milstein’s work with hybridomas earned him the 1984 Nobel Prize and the moniker, ‘Father of the Hybridoma’. Today, hybridoma technology is seen as one of the greatest breakthroughs in biotechnology of all time and holds great promise to positively impact the field of biotechnology in years to come, despite a recent downturn in usage.
Milstein’s interests were in antibodies, and the fusing of cells to create hybridomas was but an inventive step towards creating antibodies. During his experimentation, mouse myeloma cells became available to researchers and Milstein deduced that if he could fuse together a mouse myeloma cell and a mouse spleen cell, he would have a biological antibody ‘factory’. One that would last much longer and produce more reliably than the other methods in use. His plan worked, and is now the basis of a multi-billion dollar industry. Throughout the 1980s and 1990s, antibody production escalated and hybridomas became a ubiquitous part of antibody production.
Bioproducts and bioprocesses facilitated by the hybridoma platform
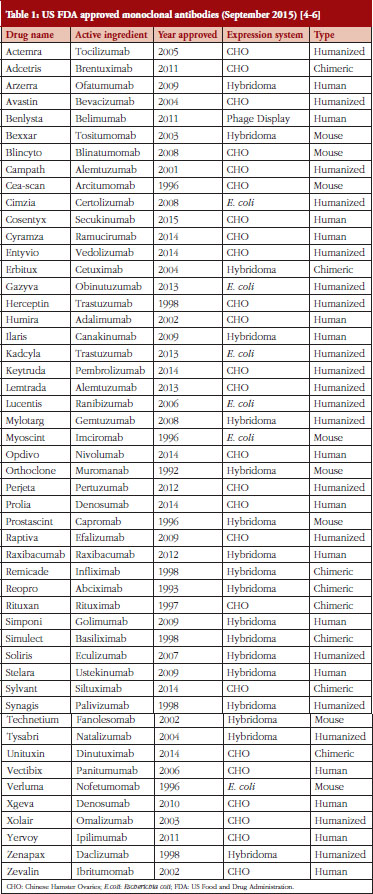
Hybridomas are used to produce monoclonal antibodies used in the prevention, diagnosis and treatment of diseases such as cancer, multiple sclerosis, heart disease, inflammatory diseases, macular degeneration, transplant rejection, and viral infections [3]. Other systems for producing monoclonal antibodies have been developed since Milstein’s pioneering work. Monoclonal antibodies (mAbs) are also made using Chinese Hamster Ovaries (CHO), yeasts, insects, plants, bacteria and phages [3]. CHO is the most popular method of mAb production due to cheaper costs, favourable approval history and reliability in the lab [3]. Hybridomas could soon see increased use in mAb production as improvements are made to the hybridoma platform [4–6], see Table 1.
Since their initial creation, hybridomas have been widely employed in the commercial production of mAbs. Initially, the main difficulty with hybridomas was in generating highly specific human antibodies when using murine (mouse) cells [7]. Hybridomas seemed fated to the production of lab-grade animal antibodies until researchers produced human hybridomas in the 1980s. Human hybridomas showed great promise in the production of human mAbs, but the technology languished due to the difficulty in obtaining starting materials and lack of technology needed for the intricate work [8]. In the 1990s, cloning techniques were refined offering new advances for human hybridoma technology. Recently, developments in single cell sorting and high throughput methods for working with small sample sizes have again breathed new life in human hybridoma technology.
The developmental studies Hybridoma Bank
The National Institutes of Health established the Developmental Studies Hybridoma Bank in 1986 as a national biotechnology resource. Their mission is to distribute hybridomas and mAbs to the scientific community. Presently, hybridomas cost US$35. The Developmental Studies Hybridoma Bank has developed seven hybridoma lines and they have an extensive antibody collection, containing 2,649 mAbs, available to researchers [9].
Hybridoma creation
Hybridomas are created in a six-step method which can also be described as ‘phases of production’, with upstream and downstream processes, as seen in all bioprocessing. The upstream phases consist of preparatory actions performed on the mice for antibody development and on the myeloma cells to create a unique fusion partner. Additional upstream processes result in the fusion of the myelomas with spleen cells, and stages of post-fusion growth. Once the cell line is immortalized, fed and nourished, downstream processes begin. Downstream processing results in long-term hybridoma growth and antibody harvesting. This entire process is completed in six stages [3]:
- Mice are injected with cells or antigens for 2–3 weeks to cause an immune response.
- The mice are tested for desired antibodies and euthanized.
- Cells from the mice spleens are extracted.
- The antibody-producing spleen cells are fused with myeloma cells by a variety of methods, becoming hybridomas.
- The hybridomas are selected singly, cloned and grown in a growth medium.
- When the hybridomas exhibit desired traits, they are harvested and transferred to long-term growth medium and kept as an immortal cell line where harvesting of antibodies occurs.
Once stabilized, cells are sorted according to their desired traits. This cell sorting has been traditionally accomplished using tedious flow cytometry or fluorescent labelling techniques, but recently developed high-throughput tools are emerging.
HyLiTE software
A software platform known as HyLiTE (for Hybrid Lineage Transcriptome Explorer) was launched in 2015 by the Massey University Bioinformatics Department at Palmerston North, New Zealand [10]. This program is capable of determining exact ploidy (number of sets of chromosomes in a cell) counts as well as gene expression and parental lineages in a single step based on mRNA. HyLiTE can be used to determine which hybridomas have desired characteristics, negating the need for lengthy post-fusion growth and selection stages.
The creation of hybridomas is often overlooked in research papers whose focus is on antibody production. The fusion method of choice for making hybridomas is a chemical method using polyethylene glycol (PEG). However, other methods are available that offer significant advantages over PEG.
Hybridoma fusion techniques
Cell fusion occurs naturally in most life forms during reproduction and growth phases of the cell cycle [1]. When cells fuse, their membranes, cytoplasm and nuclear machinery form a new cell capable of further growth. Cell fusion can naturally occur between cells of the same species or different species and between cells of similar or different functions [11].
A homokaryon is formed when identical cells fuse. We call the result of fusion between dissimilar cells a heterokaryon. Often the initial fusion only involves the cell membrane. After the first cellular division (mitosis), the cells are known as synkaryons in which the nuclear membrane is fused as well. When cells with different numbers of chromosomes fuse, the resultant cell is unstable and further mitosis results in chromosome loss. This presents a unique challenge to scientists engineering hybridomas [1].
Producing stable hybridomas is crucial. The myeloma cells used have four sets of chromosomes (tetraploid) and the spleen cells have two sets (diploid). The resulting hybridoma cells can vary in ploidy [12]. After several weeks of downstream processing, the hybridoma DNA stabilizes [13]. Here we describe the standard fusion techniques (viral, PEG and electrofusion) which rely on random cell pairings, unstable cell contact and low yields, as well as two newly devised methods (gold nano-particle laser and distortion-based).
Viral fusion methods
In 1960, scientists learned to effectively fuse cells using the Sendai virus, a respiratory tract virus that affects mice. Sendai virus fuses cells by the secretion of fusion proteins that are normally used by the virus to fuse with, and gain entry into a cell. Two cells held in close proximity with cell fusion proteins will also fuse. Other viruses, or even extracted fusion protein, may also be used to fuse cells for hybridoma production, however, antibody secretions from the fused hybridomas can vary with these other options leading researchers to mainly choose the Sendai virus [14]. Sendai virus is commercially available and still used in experiments with hybridoma production. The efficiency seen with Sendai virus is very low, with tens of thousands of cells needed to produce each viable hybridoma [14].
PEG fusion methods
Considered more reliable than using the Sendai virus, PEG has become the preferred method for fusing cells in hybridoma production [15]. Using PEG is a one-step process of simply placing the cells to be fused into a flask containing PEG and shaking [1]. PEG dehydrates the cells and fuses cell membranes as well as intracellular membranes [15]. A disadvantage to using PEG is that it causes unwanted fusions to occur [16]. PEG fusion products must be carefully assayed over the course of several weeks in the post-fusion phase to ensure the desired hybridomas are produced. It is estimated that only one in every 10,000 PEG fusion attempts leads to hybridoma formation [14]. Additionally, PEG fusion is not easily reproducible and different cell types act differently when exposed to PEG making experimentation and scale-up difficult [14].
Cell electrofusion methods
First used in 1994, and with a poor survival rate, electrofusion techniques have benefitted greatly from newly devised microfluidics platforms of the past several years [17]. Advances in bioinformatics have also made electrofusion a more precise method [17]. Electrofusion is accomplished by applying carefully controlled bursts of electricity to cells in suspension. The cell surface is first prepared through electroporation and then the cells are brought into close contact using the magnetic properties of dialelectrophoresis [17]. Properly designed, electrofusion results in a cell with a fused membrane and cytoplasm. Modern electrofusion produces more reliable fusion than PEG or Sendai virus techniques, but is not widely used due to its complexity and the technical skill required [17].
Laser fusion methods
Cell fusion using laser technology was first used in the 1980s with good results, but achieved no greater successful fusions than viruses, PEG or electrofusion [18]. Laser fusion technology is based on the principle that cells held in close proximity and bombarded with UV light will fuse [18]. Techniques available to researchers in the 1980s offered little control of the system and the fusions most often failed. An experimental technique was released in 2015 from the University of Copenhagen, Denmark. This novel fusion system is based on laser application of near infrared (NIR) light pulsed into cells held in close proximity to gold nano-particles [18]. Optically heated gold nano-particle fusion appears to offer the greatest success yet seen in cell fusion.
Deformity-based microfluidic fusion method
This method of cell fusion may revolutionize the hybridoma platform [19]. A major drawback to all other techniques is the lack of cell-to-cell contact required for proper fusion. Using precisely engineered microfluidics channels, single cells can be captured and paired, then squeezed into close contact for fusion, all within a single ‘lab-on-a-chip’ device. Other methods rely on random pairings and high numbers of fusions, with lengthy post-fusion screening steps to determine suitability for mAb production. The deformity-based microfluidic technique allows for precise control of the fusion partners and holds the resultant hybridoma in a growth medium while it is checked for proper fusion and post-fusion secretions. This is considered a high throughput device and is compatible with several different fusion techniques including viral, PEG, electrofusion, and laser. Using this platform, cell fusion efficiency up to 95% has been achieved, contrasted with an estimated 0.01% efficiency of PEG methods [19].
The importance of hybridomas in antibody therapy
Downstream processes of the hybridoma platform include the production of mAbs. Antibodies were first described in the late 1800s. An antibody (Ab), also known as an immunoglobulin (Ig), is a Y-shaped protein produced naturally by most animals. Antibodies are secreted from B cells in response to a pathogen’s specific molecular signature, known as an antigen.
Cesar Milstein and other researchers pioneered antibody therapies for use in cancer and immune system disorders in the 1960s. They first used animal models for antibody production, but these generally caused unwanted immune reactions when administered to human patients [1]. When injected into a patient, antibodies can help the immune system fight disease. The first antibodies produced by researchers were made by injecting animals with antigens and collecting serum containing antibodies. These are known as polyclonal antibodies (pAb) as they produced naturally from multiple immune B cells. PAbs are used mainly in experiments and diagnostics, and have seen some success in treating human disease.
Mabs – monoclonal antibodies are a completely natural antibody produced in laboratory experiments. MAbs are highly specific immune cells produced by cloned parent cells carefully selected for their antibody secretions. Hybridoma technology was instrumental in the early work on mAbs. MAbs have enjoyed great success in treating human disease with 50 mAbs achieving US Food and Drug Administration (FDA) approval for treatment of various diseases, see Table 1, and an estimated 70 mAbs by 2020 representing a US$75 billion industry [5]. MAbs also find use throughout the fields of molecular biology and biotechnology as tools for detecting and purifying molecular substances. The most beneficial use of hybridomas is the production of antibodies made from human cells.
Humanized and fully human mAbs – human hybridomas offer many benefits over CHO and phage-display technologies for mAb production. Their human origins preserve human DNA sequences and the pairing of antigens/antibodies can be accomplished with no genetic modifications [12]. Additionally, the possibility of contamination risk is lower using human hybridomas [12]. Animal viruses are not always apparent in animal model hybridomas but have been known to cause complications when used in human applications [12]. Both CHO and phage platforms have issues that could be resolved by the use of human hybridomas.
The difference between ‘fully human’ and ‘humanized’ hybridomas is that the fully human variety is made from human spleen cells fused with human myelomas. The humanized hybridomas may use an animal for one, or both, and may involve genetic engineering to make the animal more human-like [12]. In terms of safety and efficacy, there is little difference between humanized and fully human antibodies produced by hybridomas [12]. Fully human hybridomas are more difficult to produce because there is a scarcity of human spleen cells producing desired antibodies. In animal models, the animal is injected with an antigen to cause a secretion of a specific antibody.
Trends that may revolutionize the hybridoma platform
Zhang’s 2012 review of hybridoma usage described a ‘remarkable and indispensable platform for generating high quality monoclonal antibodies’ [20]. Zhang further noted that, ‘… the ‘old-fashioned’ hybridoma technology will open up new avenues for more effectively generating large panels of high quality and fully human mAbs’. Since Zhang’s 2012 review, new technologies have become available to researchers that stand to take the hybridoma platform out of the dark ages: HyLiTE software, gold nano-particle laser cell fusion and distortion-based microfluidics. Zhang’s review focused on 40-year-old PEG technology for the fusion steps and standard techniques for screening (ELISA, flow cytometry, fluorescence-activated cell sorting, and immunohistochemistry) that have been in use since the early 1980s [21–24]. Deformity-based microfluidic fusion methods of fusion [19] and gold nano-particle laser cell fusion techniques [18], available only since 2014, are vast improvements over PEG fusion, possibly raising efficiency rates for viable hybridomas from ~1 in 10,000 cells to ~9,500 in 10,000 cells [14, 19]. Post-fusion screening using HyLiTE software could eliminate the lengthy screening steps described by Zhang by producing ‘tables of homeologue expression data from raw mRNA read files in a single step’ [10].
Discussion and conclusions
Hybridoma technology often gets lost amidst larger discussions on mAbs in scientific papers. Outside of a few papers from the 1980s and Zhang’s 2012 review [20], no comprehensive reviews of hybridoma fusion techniques, selection of fusing partners, or post-fusion hybridoma cell selection techniques exist. Researchers who wish to study mAb production could benefit from considering the use of hybridomas for their experiments. Pharmaceutical companies looking to produce new classes and types of mAbs could benefit from using a hybridoma platform over the industry standard CHO, E. coli, and phage-display techniques. FDA has shown great willingness to approve new biologicals made from the stable hybridoma platform.
There are 50 FDA approved mAbs on the market today, of these, 18 are produced using hybridoma technology. The possibility of producing biosimilars with hybridomas to compete with existing mAbs should not go unnoticed by enterprising small-capital drug companies in light of recent developments on the hybridoma platform. Deformity-based microfluidics, gold nano-particle laser fusion and HyLiTe software for cell selection are now available for labs to use. Combined, these technologies could revolutionize the mAb industry or lead to breakthroughs in humanized or fully human mAbs.
Hybridoma technology, though 40 years old, holds promise for great impact on future biotechnology industry as a platform for drug discovery and development. As bioinformatics become more powerful and nanotechnology expands into biologicals, the possibilities of using hybridomas to study cellular interactions on a scale never before envisioned becomes a reality. Researchers in all areas of molecular biology can look to the hybridoma for clues related to cell fusion, allopolyploids, or gene expression in fused cells. There is a need for a thorough review of the hybridoma platform in light of new technologies.
|
Glossary ANTIBODIES: Immune system proteins that seek out and destroy antigens. Antibodies are specific in that each antibody binds to a particular antigen. ANTIGENS: Substances that induce the production of antibodies. Antigens are usually foreign objects such as bacteria, viruses and infectious agents. Cross-species proteins can be used as antigens. HYBRIDOMA: An immortal cell line made by fusing an antibody-producing spleen or B cell with a myeloma cell. Typically, the B cell is from a mouse that has been immunized with a human antigen. The hybridoma possesses the antibody-producing traits of the spleen (B) cell with the immortality of the myeloma cell, resulting in a cell line that can be cultured permanently and produce mass quantities of an antibody. MYELOMA: A bone marrow tumour that will grow permanently in cell culture. Myeloma (immortal) cells are fused with antibody-producing mammalian (mortal) cells to produce hybridomas. POLYCLONAL ANTIBODIES: Highly selective proteins produced naturally by the immune system for recognizing and binding to a specific antigen. These are the mechanism for naturally triggering an immune response. MONOCLONAL ANTIBODY: A single clone of a single antibody that is produced in a lab. There are four types of monoclonal antibodies: murine antibodies, chimeric antibodies, humanized antibodies, and fully human antibodies. These are distinguishable by the percentage of mouse to human parts in the antibodies. MURINE ANTIBODY: 100% mouse DNA. Used for transplant rejection and colorectal cancer, can cause serious side effects. Possess weak ability to interact with the human immune system. CHIMERIC ANTIBODY: Approximately 30% mouse DNA. A genetically engineered combination of mouse antibodies with parts of a human antibody. These show reduced effectiveness and may cause serious side effects. HUMANIZED ANTIBODY: No more than 10% mouse DNA. Humanized antibodies were produced to avoid the unwanted responses seen with mouse and chimeric antibodies. Humanized antibodies show minimal unwanted response of the human immune system. FULLY HUMAN ANTIBODY: 0% mouse DNA. The terms ‘fully human’ and ‘human’ antibody refer to antibodies derived from transgenic mice with human antibody genes or from human cells. ‘Fully human’, ‘human’ and ‘humanized’ antibodies show equal efficacy and safety. |
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
1. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495-7.
2. Taniguchi M, Miller JFAP. Specific suppressor T cell hybridomas. In: Melchers F, Potter M, Warner NL, editors. Lymphocyte Hybridomas. Springer Berlin Heidelberg; 1979. p. 212-16.
3. McDonnell S. Production of antibodies in hybridoma and non-hybridoma cell lines. In: Mohamed Al-Rubeai, editor. Animal cell culture: cell engineering 9. Springer International Publishing Switzerland; 2015. p. 65-88.
4. Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4(3):413-5.
5. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9-14.
6. U.S. Food and Drug Administration. FDA approved drug products [homepage on the Internet]. [cited 2016 Jan 8]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
7. Holzapfel BM, Wagner F, Thibaudeau L, Levesque JP, Hutmacher DW. Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells. 2015;33(6):1696-704.
8. Ying T, Li H, Lu L, Dimitrov DS, Jiang S. Development of human neutralizing monoclonal antibodies for prevention and therapy of MERS-CoV infections. Microbes Infect. 2015;17(2):142-8.
9. Developmental studies Hybridoma Bank at the University of Iowa. Monoclonal antibodies for use in research [homepage on the Internet]. [cited 2016 Jan 8]. Available from: http://dshb.biology.uiowa.edu/
10. Duchemin W, Dupont PY, Campbell MA, Ganley AR, Cox MP. HyLiTE: accurate and flexible analysis of gene expression in hybrid and allopolyploid species. BMC Bioinformatics. 2015;16:8.
11. Ringertz NR, Robert E. Savage. Cell hybrids. Academic Press; 2014.
12. Stacey GN, Byrne E, Hawkins JR. DNA profiling and characterization of animal cell lines. Methods Mol Biol. 2014;1104:57-73.
13. Kim HY, Stojadinovic A, Izadjoo MJ. Immunization, hybridoma generation, and selection for monoclonal antibody production. Methods Mol Biol. 2014;1131:33-45.
14. Smith SA, Crowe JE Jr. Use of human hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr. 2015;3(1):AID-0027-2014.
15. Stech M, Kubick S. Cell-free synthesis meets antibody production: a review. Antibodies. 2015;4(1):12-33.
16. Oshimura M, Uno N, Kazuki Y, Katoh M, Inoue T. A pathway from chromosome transfer to engineering resulting in human and mouse artificial chromosomes for a variety of applications to bio-medical challenges. Chromosome Res. 2015;23(1):111-33.
17. Dura B, Voldman J. Microfluidic systems for cell pairing and fusion. Methods Mol Biol. 2015;1313:73-94.
18. Rørvig-Lund A, Bahadori A, Semsey S, Bendix PM, Oddershede LB. Vesicle fusion triggered by optically heated gold nanoparticles. Nano Lett. 2015;15(6):4183-8.
19. Dura B, Liu Y, Voldman J. Deformability-based microfluidic cell pairing and fusion. Lab Chip. 2014;14(15):2783-90.
20. Zhang C. Hybridoma technology for the generation of monoclonal antibodies. Methods Mol Biol. 2012;901:117-35.
21. Noteboom WD, Knurr KE, Kim HS, Richmond WG, Martin AP, Vorbeck ML. An ELISA for screening hybridoma cultures for monoclonal antibodies against a detergent solubilized integral membrane protein. J Immunol Methods. 1984;75(1):141-8.
22. Andreeff M, Bartal A, Feit C, Hirshaut Y. Clonal stability and heterogeneity of hybridomas: analysis by multiparameter flow cytometry. Hybridoma. 1985;4(3):277-87.
23. Parks DR, Bryan VM, Oi VT, Herzenberg LA. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979;76(4):1962-6.
24. Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305:537-40.
|
Author for correspondence: Tim Steele, BS, University of Maryland University College, Biotechnology Department, 3501 University Boulevard East, Adelphi, MD 20783, USA |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2016 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.



Very detailed article. Can you explain what all patenting issues could arise in the case of biosimilar medicines?