Biologicals dominate Europe’s best sellers
Published: 2015/03/18
The landscape of blockbuster medicines in Europe has been changing over the last years from small molecule drugs to biologicals.
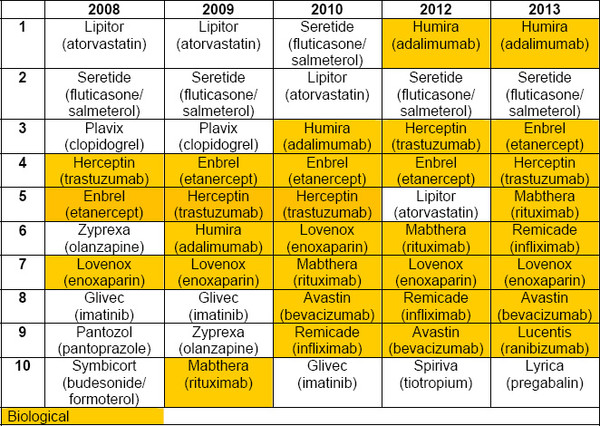
Biologicals now dominate the top 10 list of best-selling medicines in Europe, with eight of the top 10 best-selling drugs now being biologicals. This compares to only three biologicals that made the top 10 list back in 2008, see Table 1.
Table 1: Top 10 best-selling drugs in Europe 2008–2013
Source: IMS Health, MIDAS, MAT June 2013
The patents on all of these blockbuster biologicals have already expired or expire by 2022 at the latest [1], making them targets for biosimilars developers.
There are also more than 200 new biologicals in the pipeline (phase II to be registered), all of which could also be future targets for biosimilars [2].
Related article
The global biologicals market
References
1. GaBI Online – Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Jun 27]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
2. GaBI Online – Generics and Biosimilars Initiative. Biotech pipeline and biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Jun 27]. Available from: www.gabionline.net/Biosimilars/Research/Biotech-pipeline-and-biosimilars
Source: www.gabionline.net