Biosimilar monoclonal antibodies on the horizon in Europe
Published: 2013/03/04
European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use adopted the final guideline on biosimilar monoclonal antibody (mAb) and it came into effect in December 2012. The agency is also currently reviewing two marketing authorization applications (MAAs) for the biosimilar mAb infliximab.
This highlights the growing importance of mAbs and in particular biosimilar mAbs in Europe, despite the difficulties in producing them due to their highly complex and heterogeneous structures.
In fact, since 2009, when EMA held its first workshop on biosimilar mAbs, they have become the largest class of candidate biosimilars, see Figure 1, quickly overtaking granulocyte colony-stimulating factor (G-CSF, filgrastim), in the number of Scientific Advice procedures requested by biosimilars companies.
Figure 1: Scientific Advice requested from EMA by biosimilars companies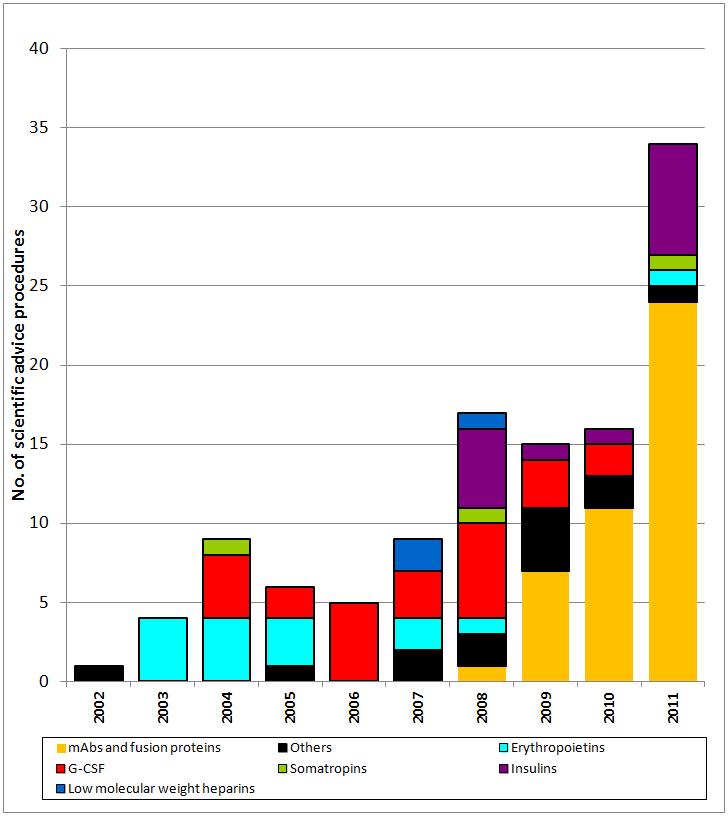
mAb: monoclonal antibody; G-CSF: granulocyte colony-stimulating factor
Source: Schneider et al.
Between 2008 and 2011 EMA reviewed 35 biosimilar mAbs in 43 Scientific Advice procedures (some companies requested Scientific Advice more than once). In fact, between 2003 and 2011, around 40% of all requests to EMA for Scientific Advice on biosimilars were for biosimilar mAbs.
Although EMA does not disclose which companies have biosimilar mAb applications under review, one of the infliximab biosimilars under review is believed to be that of biotech firm Celltrion. The Korea-based firm has already gained approval for Remsima from the Korean Food and Drug Administration and has stated that they are seeking approval in Europe.
Monoclonal antibodies represent the fastest growing segment of the biologicals market and are especially interesting for biosimilars manufacturers due to the fact that several will lose their patent protection in the next few years.
Related articles
History of biosimilar monoclonal antibodies regulation in EU
Development of biosimilars mAbs
Challenges in the development of biosimilars mAbs
Source: www.gabionline.net


