Physicochemical stability of Etoposide Accord in punctured original vials and after dilution with 0.9% sodium chloride or 5% glucose solution
Published on 2024/09/11
Generics and Biosimilars Initiative Journal (GaBI Journal). 2024;13(2):84-7.
Author byline as per print journal: Irene Krämer, PhD; Frank Erdnuess, PhD; Judith Thiesen, PhD
|
Study objectives: To determine the physicochemical stability of Etoposide Accord concentrate in punctured vials and after dilution to the concentrations 0.2 mg/mL, 0.4 mg/mL and 0.45 mg/mL in commonly used infusion fluids and containers. |
Submitted: 12 February 2024; Revised: 18 April 2024; Accepted: 26 April 2024; Published online first: 6 May 2024
Introduction
Etoposide is a semisynthetic podophyllotoxin derivative inhibiting topoisomerase II, thereby inhibiting DNA replication and ultimately causing cell death. It is indicated for the treatment of acute myeloid leukaemia, (non-)Hodgkin lymphoma, and solid tumours such as small cell lung cancer and testicular tumours. It is often used in combination with other cytotoxic agents.
Since etoposide is insoluble in aqueous solutions, the finished medicinal products contain polysorbate 80 and ethanol as solubilisers. Prior to administration, etoposide must be diluted with 0.9% sodium chloride or 5% glucose solution. While the chemical stability of diluted etoposide solutions prepared from different brand products is given for up to 61 days, physical stability is restricted due to the water insolubility of etoposide.
Precipitation of etoposide occurs depending on the etoposide concentration, storage temperature, and other physical factors, making the physical stability of etoposide solutions difficult to predict [1–4]. While Gras et al. [3] reported occurrence of precipitates in 0.4 mg/mL solutions of etoposide after 24 hours of storage at room temperature [3], D’Huart et al. showed that etoposide solutions with a concentration up to 1.26 mg/mL in 5% glucose are physicochemically stable for 61 days and etoposide solutions with a concentration up to 1.75 mg/mL in 5% glucose for 28 days when stored at 25°C [4]. Due to polysorbate 80, compatibility of etoposide products with PVC containers is limited. After 24 hours of storage in PVC bags, 20 μg/mL diethylhexylphthalat (DEHP) is leached [5].
According to the Summary of Product Characteristics (SmPC), the physicochemical in-use stability of Etoposide Accord 20 mg/mL after dilution to concentrations of 0.2 mg/mL and 0.4 mg/mL is given for up to 96 hours and 48 hours at 20°C–25°C, respectively [6]. No information is given concerning physicochemical stability at 2°C–8°C or suitable primary container material. Any unused product in the punctured original vial should be discarded [6].
Study objectives
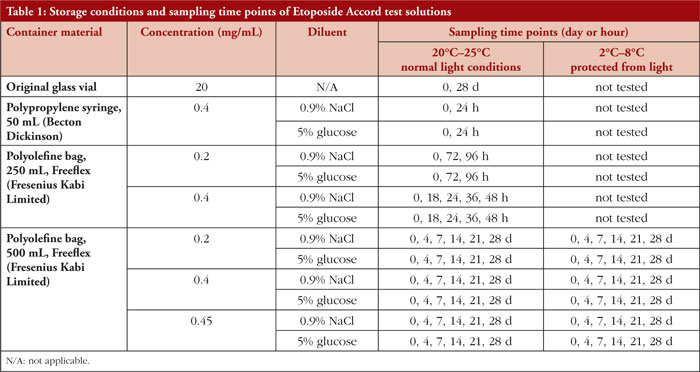
The aim of this study is to determine the physicochemical stability of Etoposide Accord 20 mg/mL concentrate for solution for infusion in punctured original vials and after dilution with 0.9% sodium chloride or 5% glucose solution at concentrations of 0.2 mg/mL, 0.4 mg/mL, and 0.45 mg/mL in 50 mL polypropylene (PP) syringes and polyolefine (PO) bags, under different storage conditions for a maximum period of 28 days.
Methods
Etoposide test solutions were prepared in accordance with the principles of Good Manufacturing Practice under EU Class A conditions. Using the European Medicines Agency (EMA) licensed Etoposide Accord 20 mg/mL concentrate (batch numbers R09903, R09905, M00391), 13 different test solutions were prepared. The test solutions were stored either light protected under refrigeration (2°C–8°C) or under normal light conditions at room temperature (20°C–25°C). Samples were taken and analysed initially (Day 0) and at predetermined time points. For detailed information, see Table 1.
The physical stability of test solutions was assessed by pH measurements (using a glass electrode, calibrated with standard buffer solutions with a pH between 1.68 and 12.45) and visual inspections for any changes in colour, clarity, or the presence of particulate matter under laboratory light against standard black and white backgrounds. Visual inspections of the concentrate and the diluted solutions should reveal clear, colourless to pale yellow, particle-free solutions. For the assessment of chemical stability, a high-performance liquid chromatography (HPLC) assay was set up and validated for linearity of analytical response and acceptable precision [7]. Etoposide Accord was defined as chemically stabile when measured etoposide concentrations remained within ±5% of the initial label claim at each time point [7].
Results
The HPLC assay was shown to be stability-indicating with no peaks overlaying the parent etoposide peak (relative retention time of etoposide-related substance A: rRt = 1.09, rRt of benzyl alcohol: rRt = 2.64).
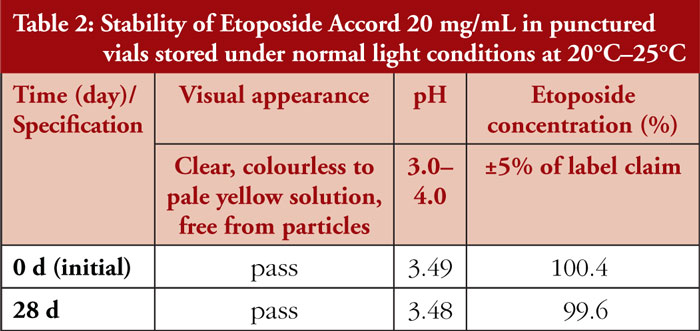
The etoposide concentrations and pH values of Etoposide Accord 20 mg/mL concentrate in punctured vials showed minor variations between Day 0 and Day 28 when stored at 20°C–25°C under normal light conditions. Neither colour change, turbidity, nor visible particles were noticeable during visual inspection, see Table 2.
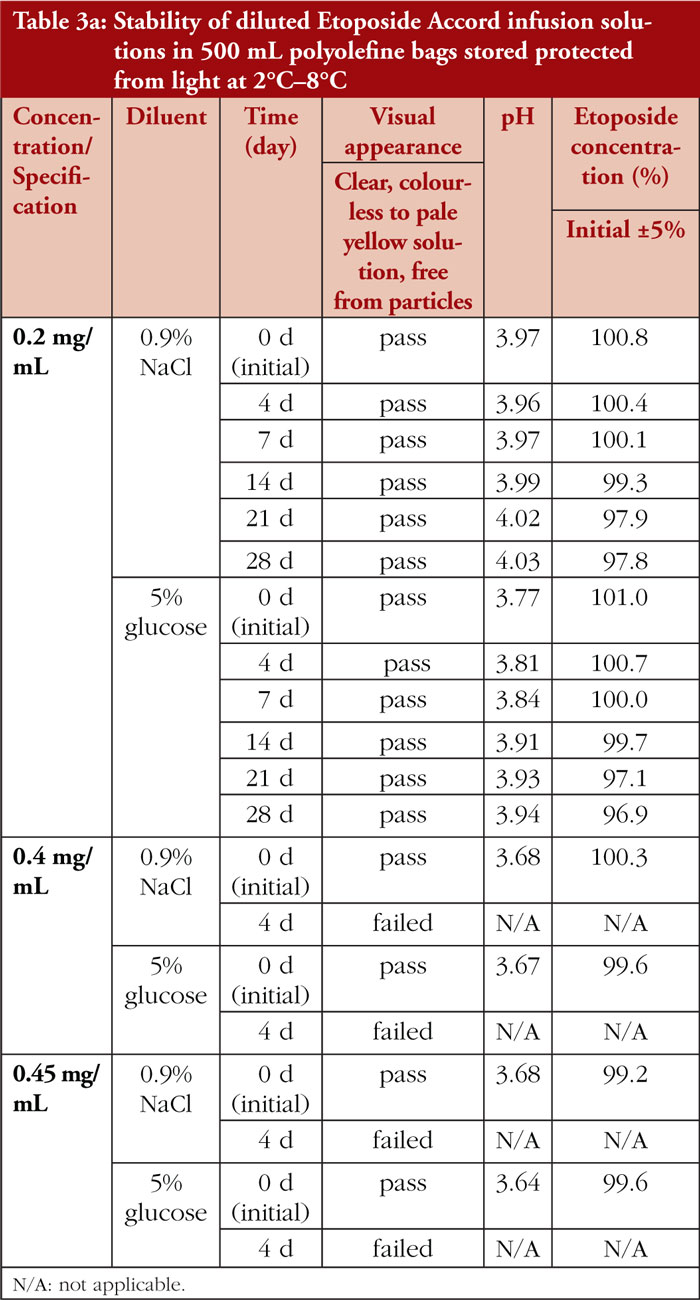
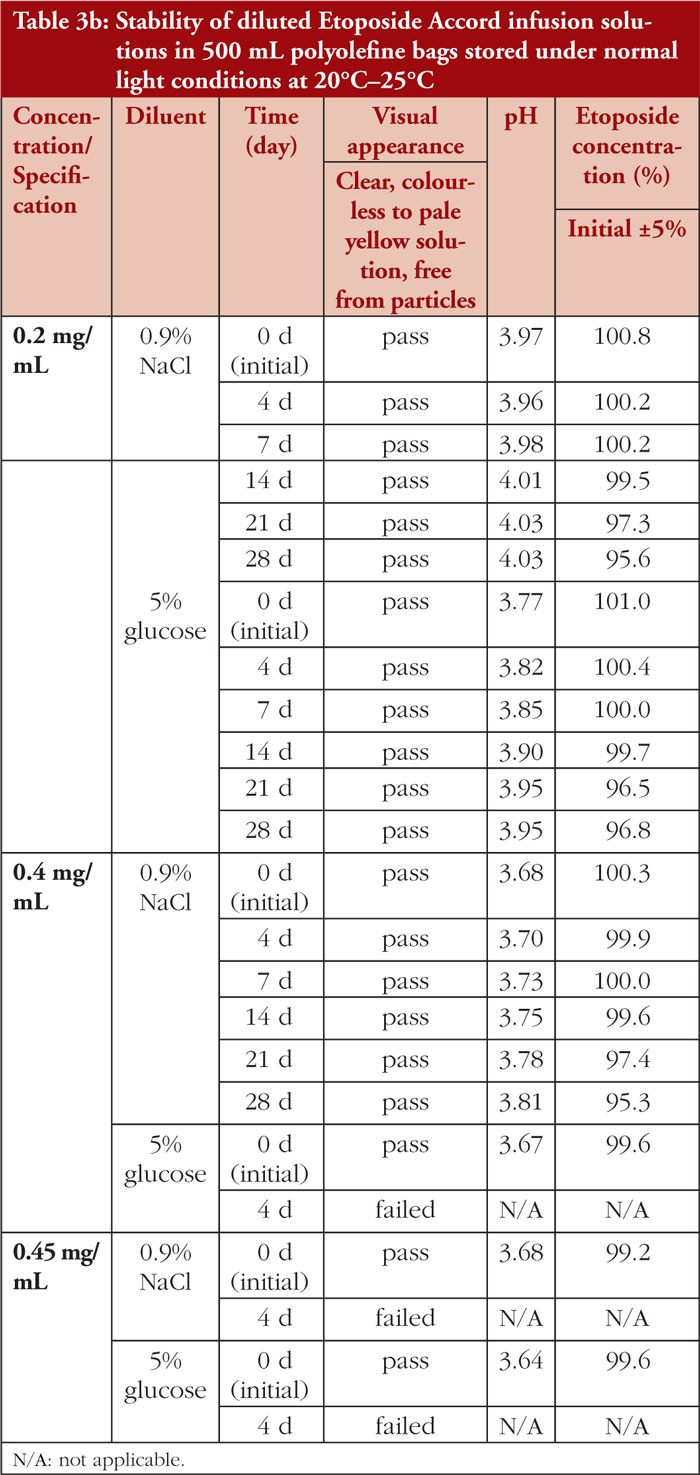
The stability of diluted etoposide test solutions was mainly dependent on the resulting etoposide concentration. Etoposide 0.2 mg/mL infusion solutions remained physicochemically stable for up to 28 days, regardless of the type of vehicle solution (0.9% sodium chloride or 5% glucose solution) or storage temperature (2°C–8°C or 20°C–25°C), as shown in Tables 3a and 3b. In etoposide 0.4 mg/mL and 0.45 mg/mL infusion solutions, particulate matter due to precipitation was noticeable at Day 4 of storage, predominantly in solutions stored under refrigerated conditions.
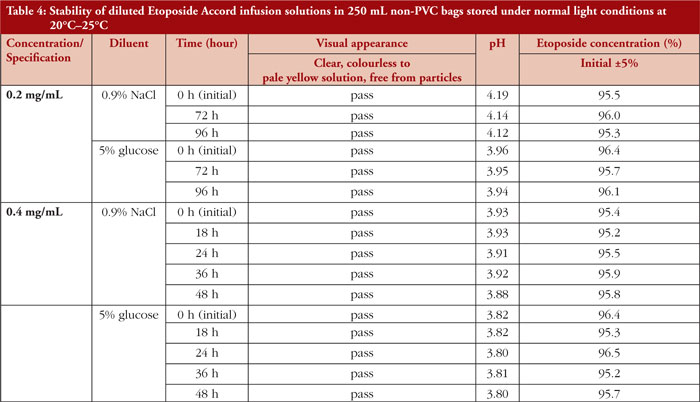
The physicochemical stability of diluted etoposide test solutions in non-PVC bags was evaluated for 96 hours at a concentration of 0.2 mg/mL and for 48 hours at a concentration of 0.4 mg/mL under normal light conditions at room temperature, independent of the vehicle solution (0.9% sodium chloride, 5% glucose solution), as shown in Table 4. No precipitation occurred during the inspection period.
In 50 mL polypropylene syringes, the physicochemical stability of diluted etoposide test solutions (0.4 mg/mL) was established for 24 hours at room temperature under normal light conditions, as shown in Table 5.
Conclusion
Etoposide Accord solution concentrate 20 mg/mL has been revealed to be physicochemically stable for 28 days after the first opening when stored under normal light conditions at room temperature.
After dilution with 0.9% sodium chloride or 5% glucose to a concentration of 0.2 mg/mL, the physicochemical stability of etoposide infusion solution was proven for up to 28 days, independent of storage temperature. Neither a change in visual appearance nor visible particles was noticeable. The stability of etoposide solutions 0.4 mg/mL and 0.45 mg/mL is limited to a maximum of 4 days. Precipitation occurred already at Day 4 (the first predetermined test time point) of storage, especially in solutions stored refrigerated. These results are in line with published data reporting appearance of particulate matter in etoposide solutions at concentrations higher than 0.2 mg/mL within 1 to 3 days of storage [1–3].
Residues in the punctured original glass vials can be used cost-effectively for up to 28 days when stored at room temperature. Diluted infusion solutions with etoposide concentrations of up to 0.2 mg/mL can be prepared in advance by pharmacy-based cytotoxic preparation units and used over a period of 28 days. Diluted infusion solutions with etoposide concentrations higher than 0.2 mg/mL should be stored at room temperature for a maximum of 96 hours. In any case, etoposide infusion solutions must be inspected for particulate matter prior to administration.
Analysis was performed and documented by an accredited external laboratory. Results were carefully checked for plausibility and cautiously interpreted.
Funding sources
This study was funded by Accord Healthcare.
Competing interests: The authors Irene Krämer, Frank Erdnuess, and Judith Thiesen have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Authors
Professor Irene Krämer, PhD
Frank Erdnuess, PhD
Judith Thiesen, PhD
Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany
References
1. Barthes DM, Rochard EB, Pouliquen IJ, Rabouan SM, Courtois PY. Stability and compatibility of etoposide in 0,9% sodium chloride injection in three containers. Am J Hosp Pharm. 1994;51(21):2706-9.
2. Lepage R, Walker SE, Godin J. Stability and compatibility of etoposide in normal saline. Can J Hosp Pharm. 2000;53:338-45.
3. Gras C, Sautou-Miranda V, Bagel-Boithias S, Brigas F, Chopineau J. Compatibility of etoposide solution with polyvinyl chloride and low density polyethylene containers and its stability in different storage conditions. Eur J Hosp Pharm. 2002;2:33-40.
4. D’Huart E, Vigneron J, Lider P, Demoré B. Physicochemical stability of etoposide diluted at range concentrations between 0.38 and 1.75 mg/mL in polyolefin bags. Eur J Hosp Pharm. 2020;27(1):43-8.
5. Demoré B, Vigneron J, Perrin A, Hoffman MA, Hoffman M. Leaching of diethylhexyl phthalate from polyvinyl chloride bags into intravenous etoposide solution. J Clin Pharm Ther. 2002;27(2):139-42.
6. Accord Healthcare Limited. Summary of product characteristics for Etoposide Accord 20 mg/mL concentrate for solution for infusion. Available from: https://cdn.accord-healthcare.com/ie/public/spc/downloadspc_etoposide_20mg.pdf
7. Accord Healthcare Limited. Data for HPLC assay and acceptance criteria on file; 21-07-2015.
|
Author for correspondence: Judith Thiesen, PhD, Department of Pharmacy, University Medical Center of the Johannes Gutenberg University Mainz, 1 Langenbeckstraße, DE-55131 Mainz, Germany |
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2024 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.




Dear colleague,
Congratulations for this paper.
Is it possible to obtain table 1 to 5 because they are not accessible
Many thanks,
Pr JD HECQ
Etoposide Accord
Dear Professor JD HECQ,
We greatly appreciate your kind feedback. The missing tables 1 to 5 from the Etoposide Accord article have been posted online.
Thank you for your interest in GaBI. We hope you enjoy the high-quality information and content available under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office