‘Similar biologics’ approved and marketed in India
Published on 2013/02/07
Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):50-1.
There have been established guidelines for approving generic versions of small molecule chemical drugs in India for some time already. However, no specific guidelines for ‘similar biologics’, as the Indian regulatory authorities call these products, have existed in India until recently. This has been the case despite the fact that the requirements for granting regulatory approval for such ‘similar biologics’ required more data than for a simple generic drug application [1].
India announced the release of draft regulatory guidelines for ‘similar biologics’ at the BIO industry conference in Boston, USA, on 19 June 2012, and implemented them from 15 September 2012. The guidelines outline a simple abridged procedure for evaluation of ‘similar biologics’ which have been approved and marketed in India, Europe or USA for more than four years [2].
Biosimilars are firmly established in the EU as copy biologicals with a clear and effective route for approval [3]. It should be noted, however, that ‘similar biologics’ approved and marketed in India have not been subjected to the same rigorous controls and have not necessarily been compared in direct clinical trials to the reference product as is demanded in Europe by the European Medicines Agency. They should not therefore be referred to as biosimilars according to the definition proposed by EMA:
‘A biosimilar is a copy version of an already authorized biological medicinal product with demonstrated similarity in physicochemical characteristics, efficacy and safety, based on a comprehensive comparability exercise’ [4].
The Central Drugs Standard Control Organization is responsible for the approval, i.e. marketing authorisation of medicinal products, including these so-called ‘similar biologics’, in India.
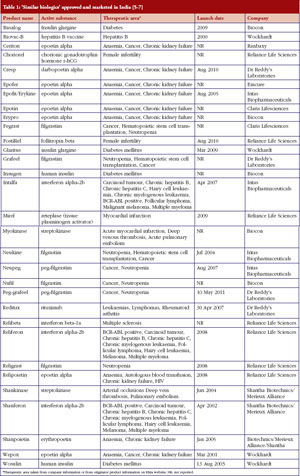
India has, by far, demonstrated the greatest acceptance of ‘similar biologics’. According to our research at GaBI Online, the first ‘similar biologic’ was approved and marketed in India for a hepatitis B vaccine in 2000. In recent years over 50 biopharmaceutical products have been approved for marketing in India, with more than half of them being ‘similar biologics’ [5], see Table 1.
Competing interests: None.
Provenance and peer review: Article prepared based on extensive research; internally peer reviewed.
Michelle Derbyshire, PhD, GaBI Online Editor
References
1. Joshi SR, Biosimilar peptides: need for pharmacovigilance. J Assoc Physicians India. 2011;59 Suppl:44-7.
2. GaBI Online – Generics and Biosimilars Initiative. India releases draft ‘similar biologic’ guidelines [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Sep 21]. Available from: www.gabionline.net/Guidelines/India-releases-draft-similar-biologic-guidelines
3. GaBI Online – Generics and Biosimilars Initiative. EMA proposes more precise definition for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Sep 21]. Available from: www.gabionline.net/Biosimilars/Research/EMA-proposes-more-precise-definition-for-biosimilars
4. Wadhwa M, Thorpe R. Terminology for biosimilars-a confusing minefield. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(3-4):132-4. doi:10.5639/gabij.2012.0103-4.023
5. Jayaraman K. India’s Cipla sets sights on Avastin, Herceptin and Enbrel. Nature Biotechnol. 2010 Sep;28(9):883-4.
6. Mody R, et al. How similar are biosimilars in India? Pharmafocus Asia [monograph on Internet]. c2004–2012 Ochre media; [cited 2012 Sep 21]; Available from: www.pharmafocusasia.com/research_development/blind-comparative-study.html
7. Som N. India on biologics trail. Biospectrum. 13 Feb 2012.
For the updated version of this article, please click here.
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2013 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.



I need this pdf file
We very much appreciate your kind feedback. The PDF is available to download on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Your perspective on biologicals is refreshing and thought-provoking. Very insightful information.