Comparison of biosimilars guidelines
Published on 2013/09/30
Generics and Biosimilars Initiative Journal (GaBI Journal). 2014;3(1):36-8.
|
Abstract: |
Biosimilars are recognized around the world as safe and effective medicines. Despite this fact, they are still relatively new to most countries and make up only a small part of the biologicals market. The growing demand for ‘life-changing’ biologicals, however, is fuelling their growth.
Regulatory guidelines and standards for biosimilars have been introduced around the world but are still being developed in some countries. The clarity of these guidelines, however, is variable and the regulatory pathways are diverse, with even agreement on how to define biosimilars differing sometimes significantly between different countries and regions. Guidelines for biosimilars are constantly evolving as technology develops, but how do these guidelines differ in different regions? In this paper, biosimilars guidelines in some of the major countries/regions around the world are compared.
Europe is by far the most advanced in the regulation of biosimilars, and has the best-established framework for approval of biosimilars, being the first to create guidelines for these products. In the European Union (EU), a legal framework for approving biosimilars was established in 2003. The regulatory body for the approval of medicines in the EU is the European Medicines Agency (EMA). EMA issued its first guideline for the approval of biosimilars via an abbreviated registration process in 2005 and has since developed many general and specific guidelines for biosimilars [1].
Biosimilars regulations in Japan were issued by Japan’s regulatory authority, the Ministry for Health Labour and Welfare (MHLW), in March 2009 and follow the principles of the EU biosimilars regulations [2–4]. The Korean Food and Drug Administration (KFDA) issued a guideline regarding the regulation of biosimilar products (Guideline on Evaluation of Biosimilar Products) in July 2009 [5]. Health Canada finalized guidelines for subsequent entry biologics (SEBs) in March 2010 [6]. While global guidelines on similar biotherapeutic products were adopted by the WHO Expert Committee on Biological Standardization at its 60th meeting in October 2009 [7].
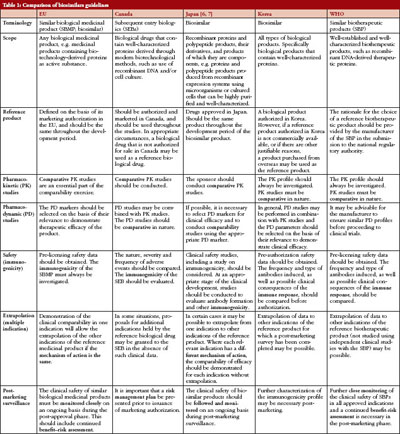
How guidelines for biosimilars compare around the world (Canada, EU, Japan, Korea and WHO) is shown in Table 1.
Table 1 shows that there are many similarities between the different countries/regions in their approach to guidelines for biosimilars. The guidelines are all based on the fact that biosimilars are intended to be used at the same dose(s) and dosing regimen(s) as the reference product. They all focus on the demonstration of (bio)similarity not individual patient benefit. All the guidelines also require an extensive comparability exercise to be carried out to ensure similar quality, safety and efficacy. The scientific principles underlying the comparability exercise required for a biosimilar are the same as that required for changes in the manufacturing process of an originator biological. Similar physicochemical characteristics are a prerequisite for reduction in non-clinical and clinical data requirements, i.e. use of the abbreviated biosimilars pathway.
The harmonization of regulatory standards for biosimilars would be of great advantage to biosimilars manufacturers. This would enable them to reduce costs and create a level playing field for manufacturers from different countries/regions. The development of a global reference product would also be of a great advantage, allowing manufacturers to reduce the number of trials required for global approval.
Global guidelines for the development of biosimilars need to be scientifically sound and in accordance with recognized strict regulatory standards, such as those of the EU and WHO. On the other hand, they also need to be flexible enough to account for differences in national regulations and market capabilities.
Competing interests: None.
Provenance and peer review: Article prepared based on extensive research; internally peer reviewed.
Michelle Derbyshire, PhD, GaBI Online Editor
References
1. Blank T, et al. Safety and toxicity of biosimilars—EU versus US regulation. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(3):144-50. doi:10.5639/gabij.2013.0203.039
2. Arato T. Regulatory guidelines for biosimilars in Japan. 10th EGA International Symposium on Biosimilar Medicines; 2012 April 20; London, UK.
3. Derbyshire M. Biosimilar development and regulation in Japan. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(4):207-8. doi:10.5639/gabij.2013.0204.055
4. GaBI Online – Generics and Biosimilars Initiative. Japanese guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Nov 12]. Available from: www.gabionline.net/Guidelines/Japanese-guidelines-for-biosimilars
5. GaBI Online – Generics and Biosimilars Initiative. South Korean guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Nov 12]. Available from: www.gabionline.net/Guidelines/South-Korean-guidelines-for-biosimilars
6. GaBI Online – Generics and Biosimilars Initiative. Canadian guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Nov 12]. Available from: www.gabionline.net/Guidelines/Canadian-guidelines-for-biosimilars
7. GaBI Online – Generics and Biosimilars Initiative. Global guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Nov 12]. Available from: www.gabionline.net/Guidelines/Global-guidelines-for-biosimilars
Disclosure of Conflict of Interest Statement is available upon request.
Copyright © 2014 Pro Pharma Communications International
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.



Any idea on when the table 1 will be uploaded? Thanks a lot.
Is it possible to get a copy of table 1? Unable to enlarge the table
please send me a printable version.
Great website you have here but I was wanting to know if you knew of any
community forums that cover the same topics discussed in this article?
I’d really like to be a part of online community where I can get
suggestions from other knowledgeable people that share the same interest.
If you have any suggestions, please let me know. Cheers!
Can you share a higher resolution version of the table?
I can’t read the table contents. Is there a bigger resolution available?
Dear Sir/Madam,
The table 1 is not visible, could you please send this review in readable format to me? I am urgently in need of it in order to complete my research.
Thanks in advance.
Kindest regards,
Melisa Şahverdi
please, Could you send me the table 1 in a readable format?
Kindly share full article in PDF and table is not visible Kindly share the table of comparison
Dear Ejaz Ahmed,
We very much appreciate your kind feedback. The print version is available on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear Ms Gillian Christians,
We very much appreciate your kind feedback. Table 1 of the article is available on the website.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear Mr Jorge Pignataro,
We very much appreciate your kind feedback. Table 1 of the article is available on the website.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear Ms Melisa Şahverdi,
We very much appreciate your kind feedback. Table 1 is available on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear Michiel,
We very much appreciate your kind feedback. Table 1 is available on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear Cao Dang,
We very much appreciate your kind feedback. You may follow GaBI on Twitter or LinkedIn.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office
Dear F,
We very much appreciate your kind feedback. Table 1 is available on the website article.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
Dear GaBi Team,
despite of your above responses, there seems to be no different, legible version of Table 1 besides the one visible on the page above. (The ‘print’ version makes it rather worse.)
It would be targeting and of great help if you could – e.g. linked from the small table picture – a bigger version with legible content.
Thanks a lot for your appreciated efforts.
With kind regards,
Matthias Brunner