What lessons can be learned from the launch of generic clopidogrel?
Published on 2012/03/05
Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):58-68.
Author byline as per print journal: Christoph Baumgärtel1, MD; Brian Godman2,3,4, BSc, PhD; Rickard E Malmstrom5, MD, PhD; Morten Andersen6, MD, PhD; Mohammed Abuelkhair7, PharmD; Shajahan Abdu7, MD; Marion Bennie8,9, MSc; Iain Bishop9, BSc; Thomas Burkhardt10, MSc; Sahar Fahmy7, PhD; Jurij Furst11; Kristina Garuoliene12, MD, PhD; Harald Herholz13, MPH; Marija Kalaba14, MD, MHM; Hanna Koskinen15, PhD; Ott Laius16, MScPharm; Julie Lonsdale17, BSc; Kamila Malinowska18, MD; Anne M Ringerud19, MScPharm; Ulrich Schwabe20, MD, PhD; Catherine Sermet21, MD; Peter Skiold22, MSc, PhD; Ines Teixeira23, BA, MSc; Menno van Woerkom24, MSc; Agnes Vitry25, PharmD, PhD; Luka Vončina26, MD, MSc; Corrine Zara27, PharmD; Professor Lars L Gustafsson4, MD, PhD
|
Introduction and study objectives: Resource pressures will continue to grow. Consequently, health authorities and health insurance agencies need to take full advantage of the availability of generics in order to continue funding comprehensive health care particularly in Europe. Generic clopidogrel provides such an opportunity in view of appreciable worldwide sales of the originator. However, early formulations contained different salts and only limited indications. Consequently, there is a need to assess responses by the authorities to the early availability of generic clopidogrel including potential reasons preventing them from taking full advantage of the situation. In addition, it is necessary to determine the extent of initial price reductions obtained in practice to guide future activities. |
Submitted: 13 September 2011; Revised manuscript received: 2 December 2011; Accepted: 5 March 2012
Introduction and study objectives
There is increasing focus on pharmaceutical expenditure globally [1], driven by factors including changing demographics and the continued launch of new premium priced medicines [1–7]. This has stimulated a number of initiatives surrounding generics, with European countries learning from each other as they continually search for additional measures to further enhance prescribing efficiency [1, 3, 4, 6, 7]. Initiatives include measures to enhance the utilisation of generics versus originators and patent protected products in the class or related class, as well as measures to obtain low prices for generics [1, 3, 4, 6–8]. This includes generic clopidogrel, with global sales of the originator at US$9.8 billon in 2009 and US$9.7 billion in 2010 [9, 10]. However, there have been concerns with different salts and indications between the originator and early generic clopidogrel formulations, which could reduce potential health authority and health insurance agency savings from the availability of generic clopidogrel. In addition in the US, the originator manufacturer also instigated a range of activities to delay the entry of generic clopidogrel. These included a recent successful and prolonged legal battle against a Canadian generics manufacturer [11, 12].
These issues regarding generic clopidogrel have arisen because manufacturers have been able to address the technicalities of Plavix’s European patent protection early by producing clopidogrel in a different salt, such as the besylate salt, and initially, only launching for secondary prevention of atherosclerotic events post myocardial infarction or post ischaemic stroke, i.e. without the acute coronary syndrome (ACS) indication [11, 13, 14].
The Swiss generics company Acino has been able to market its generic clopidogrel in Germany since August 2008. By the end of 2008, Acino’s generic clopidogrel accounted for approximately one quarter of total clopidogrel utilisation [13, 14]. Other generics versions were also launched in Austria in 2008. However, it was not until mid 2009 that EMA was able to approve various generic clopidogrel preparations through its centralised procedure [11, 13, 14]. This included more than 20 generic clopidogrel products, which contained the besilate and hydrogen sulphate salts, of which eight were approved for both indications, i.e. both secondary prevention and ACS indications [15]. However, in the UK for instance, initial generics typically only included the secondary prevention indication in their submissions [15, 16].
Health authority or health insurance agencies faced similar issues to drug licensing authorities when considering reimbursement and/or recommending the prescribing of generic clopidogrel versus the originator potentially impacting on outcomes. These included whether changing the salt would alter the rate of absorption, toxicity and stability of the active drug. In addition, efficacy questions were raised by the fact that bioequivalence studies measured only the parent compound or inactive metabolite rather than the low and transient concentrations of the active metabolite, present only briefly after dosing, as well as possible concerns with inter-patient variability [17–22]. There have also been concerns among some authorities that any putative interaction between clopidogrel and proton pump inhibitors will be less well known initially for the generic salts. These concerns were in addition to patent issues in each European country, the latter leading to widely different dates when generics become available for prescribing [3, 4]. Additionally, there have been issues regarding the functional integrity of CYP2C19 in patients as this could potentially affect the availability of the clopidogrel and hence outcomes in practice [23, 24]. As such, personalised medicine using tailored individualised antiplatelet treatment based on pharmacogenetic testing could be helpful in identifying which patients should be treated with clopidogrel and which with newer drugs such as prasugrel and ticagrelor. However, other studies have questioned this [25–29]. In any event, this should not impact on the debate of whether generic or originator clopidogrel should be prescribed. Of potential greater importance is the widely different timescales that currently exists among European countries when authorising reimbursement for generics [13, 30].
The situation for health authorities and health insurance agencies was further complicated by the EMA recall in March 2010 of clopidogrel besylate produced by Glochem Industry Ltd’s manufacturing facility in India [31–34]. The medicines concerned included Clopidogrel 1A Pharma, Clopidogrel Acino, Clopidogrel Acino Pharma, Clopidogrel Acino Pharma GmbH, Clopidogrel Hexal, Clopidogrel Ratiopharm, Clopidogrel Ratiopharm GmbH and Clopidogrel Sandoz. The marketing authorisation holder of all these products was Acino Pharma GmbH [31–34], which held the market authorisation for the majority of early generics formulations. However, Acino and other companies have been able to source generic clopidogrel from other companies to overcome possible supply problems, with multiple companies and formulations now typically available across Europe. The originator manufacturer tried to take advantage of these recalls through pointing out the known quality of Plavix [35]. The impact of this approach though was reduced in reality by EMA approval of a number of generic clopidogrel formulations from different manufacturers. In addition, European health authorities and health insurance companies are continually seeking ways to fund new premium priced drugs and increased drug volumes from ageing populations within finite resources through encouraging greater generics utilisation, Table 1 as well as references 1 and 36 contain examples of different authority approaches across Europe to enhance generics utilisation with similar approaches among managed care organisations in the US [1–4, 5–8, 36].
Consequently, the principal objective of this paper is to document health authority and health insurance agency responses to take advantage of the early availability of generic clopidogrel products. Secondly, to assess potential reasons preventing health authorities and health insurance agencies from taking full advantage of the early availability of generic clopidogrel, and potential ways to address this in the future. Finally, to determine the extent of price reductions that have been obtained by a range of countries for generic clopidogrel versus pre-patent loss originator prices in the initial months following generics availability. This aims to provide knowledge of how the future availability of generics in high expenditure areas can be accelerated, combined with measures to enhance their rapid uptake versus originators, to rapidly release valuable resources.
Methods
We first performed a literature review of English language papers in PubMed, MEDLINE and Embase between 2005 and April 2011 using the keywords ‘generic clopidogrel’. But because this resulted in only a limited number of publications, e.g. only seven relevant English language papers were cited in PubMed, the literature search was supplemented by additional information, papers and web-based articles known to the many co-authors from health authorities, health insurance agencies and their advisers from across Australia, Europe and the Middle East regarding generic clopidogrel. This information was subsequently re-confirmed with each co-author by the lead co-author Dr Brian Godman to ensure the accuracy of the data provided, hence its robustness. This is an accepted technique where there is limited information publically available to achieve study aims [2–4, 6, 7, 37–42]. No attempt was made to review the quality of the published studies using the methodology of the Cochrane Collaboration [43] in view of the paucity of peer-reviewed published studies.
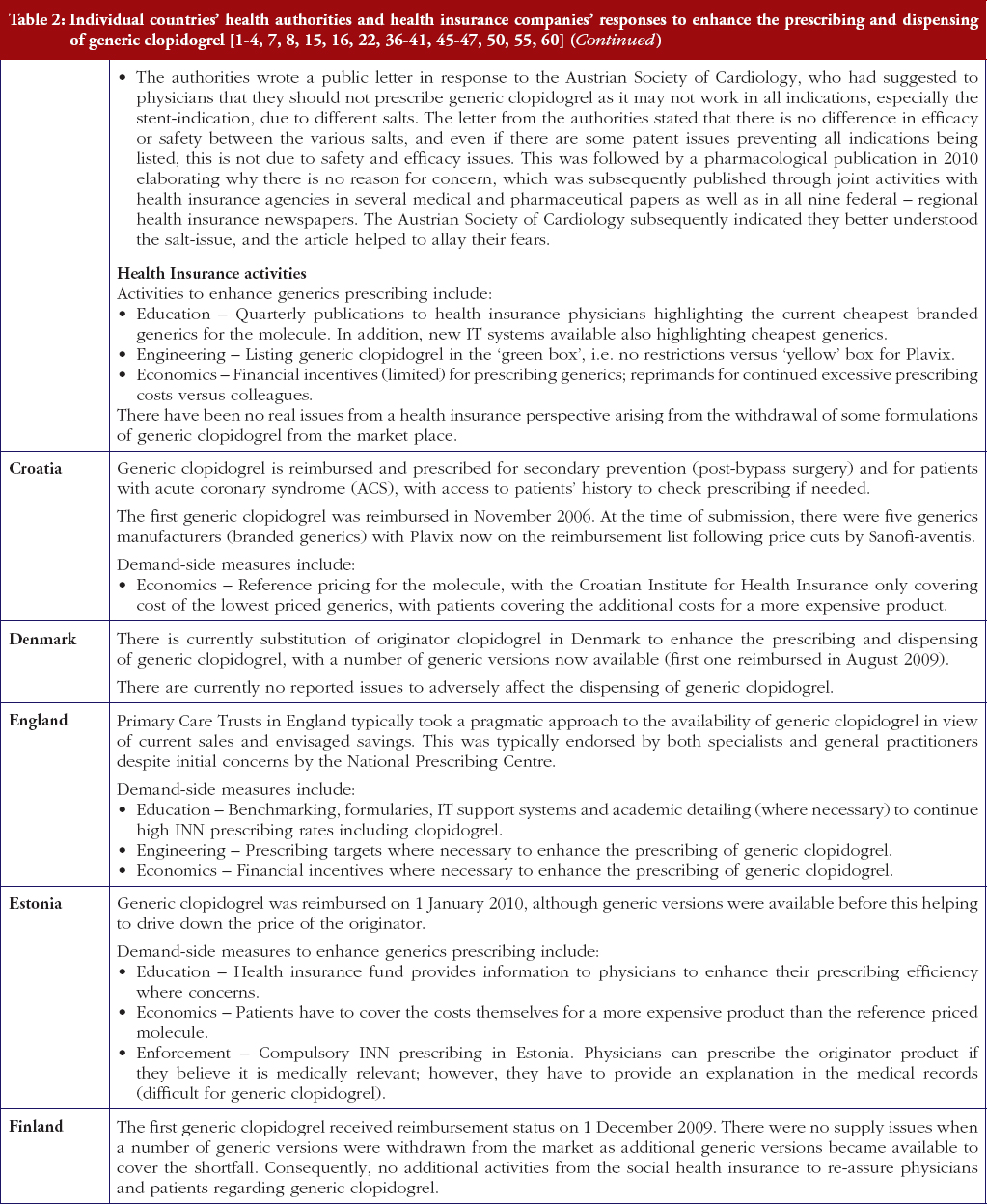
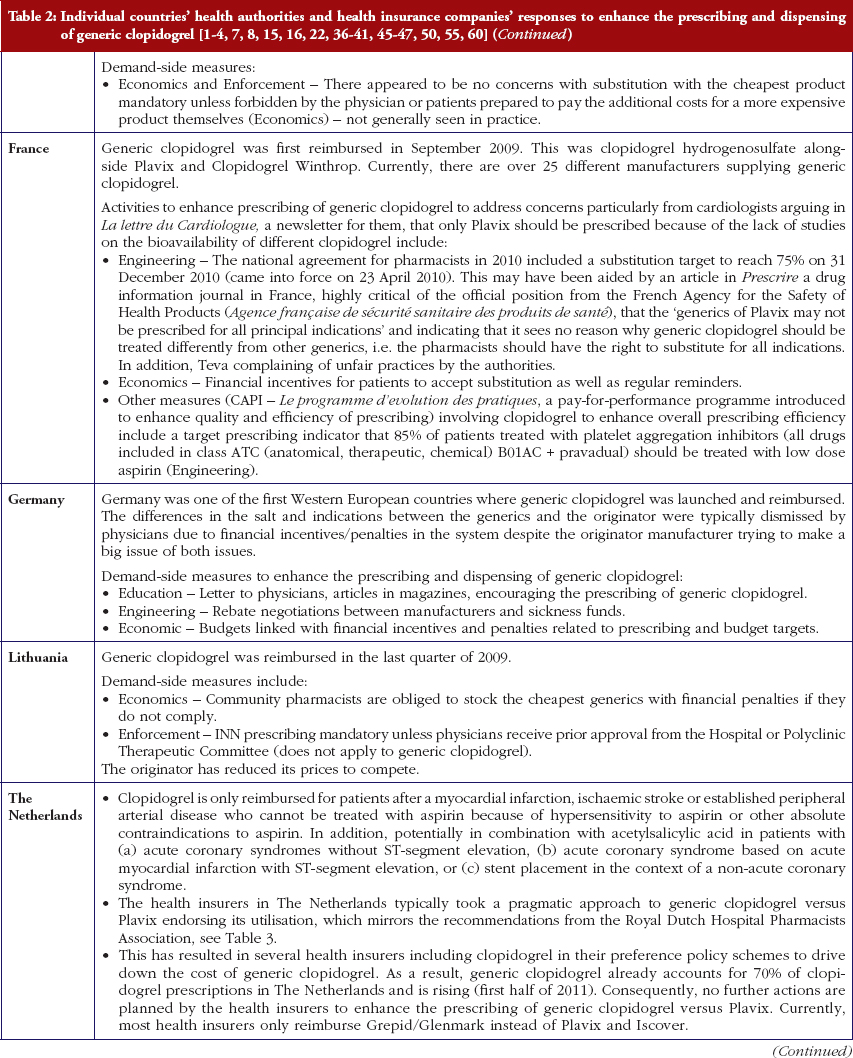
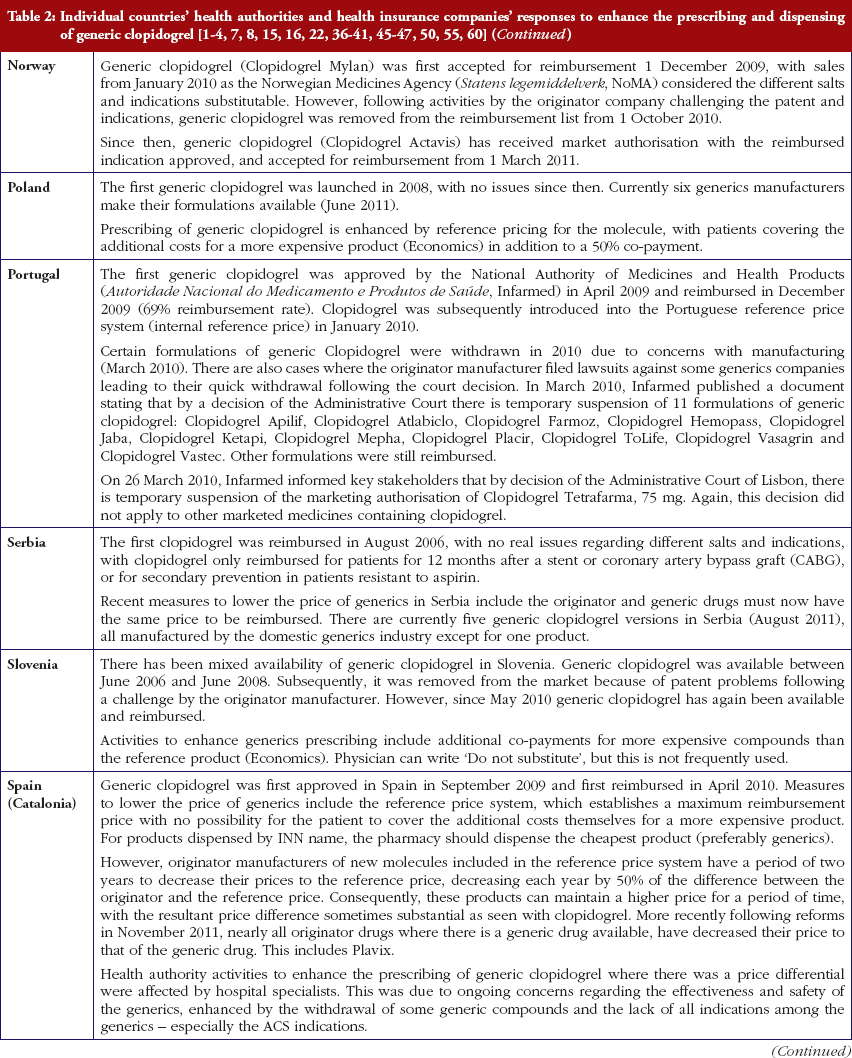
Reimbursed prices for generic clopidogrel were either provided directly from the co-authors from their own internal sources based on the 75 mg tablet (Personal communications from: Mr Iain Bishop, Mr Thomas Burkhardt, Dr Jurij Furst, Dr Kristina Garuoliene, Dr Hanna Koskinen, Mr Ott Laius, Dr Catherine Sermet, Dr Peter Skiöld, Professor Ulrich Schwabe, Dr Agnes Vitry); alternatively from administrative databases (Republic of Serbia’s Health Insurance Fund database, Dr Marija Kalaba). The findings were again validated with pertinent co-authors to ensure accuracy. Data from administrative databases included reimbursed expenditure/defined daily dose (DDD)–with DDDs defined as ‘the average maintenance dose of the drug when used on its major indication in adults’ [44]–for both the originator and generics. This approach has been successfully used in previous publications when reviewing the impact of ongoing reforms to reduce generics prices versus originators to enhance future prescribing efficiency in Europe [2–4, 6, 7, 37–40]. The countries reviewed were selected based on their different geographies, financial base for the healthcare system (taxation or insurance based) and population size to enable comprehensive comparisons of payer activities as well as reimbursed prices to provide examples to others. In addition in some countries, generic clopidogrel has only recently been reimbursed, see Table 2.
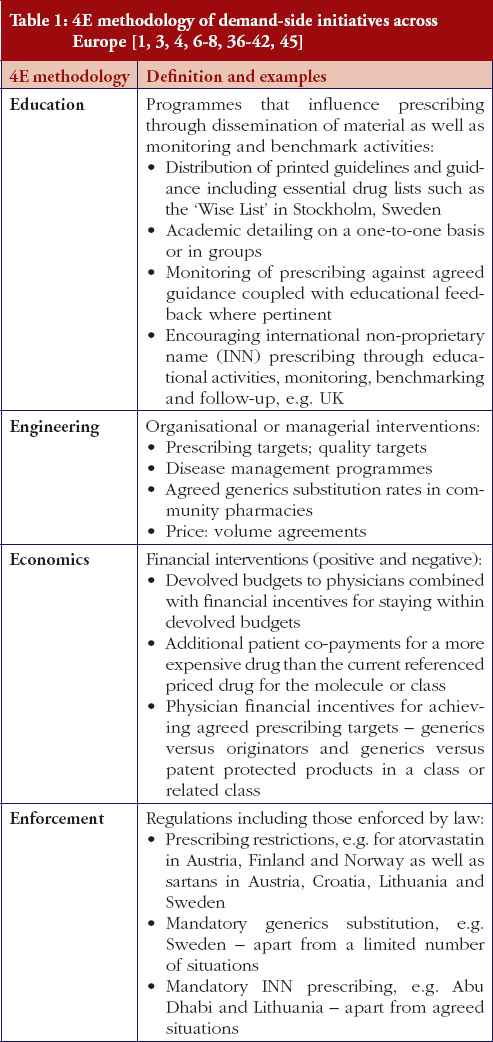
The demand-side measures initiated in each selected country to enhance the utilisation of generic clopidogrel have been taken from published sources supplemented with additional information from the co-authors. The latter approach providing most data in view of, as stated, limited available information in the public domain. Demand-side activities were again checked with pertinent co-authors to ensure the accuracy of the information provided. The various demand-side measures were subsequently collated using the 4E methodology, i.e. education, engineering, economics and enforcement, to simplify comparisons between countries, see Table 1. This approach has been successfully used in other settings to compare and contrast the influence of different demand-side interventions in practice [3, 4, 6, 38–40].


Results
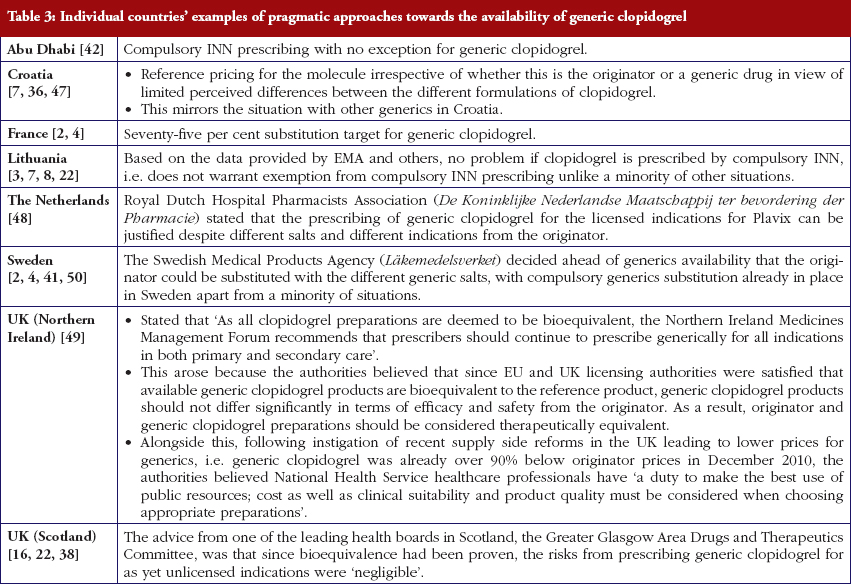
Most health authorities and insurers have adopted a pragmatic approach towards differences in the salt and indications between the generic and the originator drug to enhance the prescribing of generic clopidogrel, see Table 2; with examples of pragmatic approaches documented in Table 3. However, this has not always been possible. For example, activities in Norway, Portugal and Slovenia have resulted in all or some versions of generic clopidogrel being removed from the market place for a period of time, see Table 2.
There has also been extensive education of physicians in some European countries to allay their fears about prescribing generic clopidogrel with different salts and indications, see Table 2. As a result, utilisation of generic clopidogrel has been enhanced thereby helping health authorities and health insurance agencies gain savings from the early availability of generic clopidogrel given the global expenditure on Plavix pre-patent loss [9, 10].
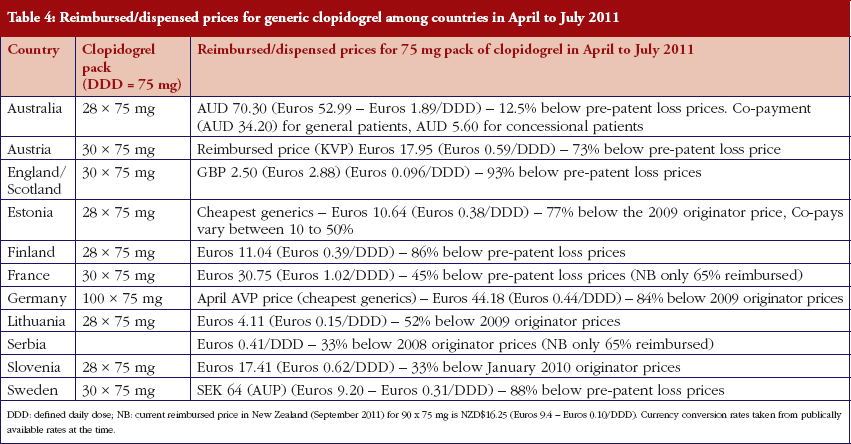
The various measures instigated among countries to obtain low price of generics [2, 4] has already resulted in appreciable price reductions in some countries. However, this was not universal with a 20-fold difference in reimbursed prices existing between countries in April to July 2011, see Table 4.
Conclusion
Health authorities and health insurance agencies have typically adopted a pragmatic approach to enhance the prescribing and dispensing of generic clopidogrel once available. As a result, valuable resources have been released from the early availability of generic clopidogrel. This is despite different salts and more limited indications initially versus the originator, coupled with the withdrawal of some formulations of generic clopidogrel from the market place due to manufacturing concerns.
Activities undertaken by health authorities and health insurance agencies to enhance the prescribing of generic clopidogrel, see Table 2, mirror those undertaken for other generics [2–4, 6, 7, 37–40]. They also included extensive education among key stakeholder groups in some countries to enable health authorities and health insurance agencies to fully realise the finan-cial benefits from the early availability of generic clopidogrel. However, activities in some countries have not always been possible following successful challenges to the availability of generic clopidogrel, which led to the removal of all or some formulations for a period of time, see Table 2.
It may well be in the long term that compliance is a greater issue to maximise outcomes from clopidogrel than any perceived differences in bioavailability between formulations, mirroring the situation with other cardiovascular drugs [51]. Consequently, some of the resources released from the availability of generic clopidogrel could be used to address this issue to maximise the health gain from clopidogrel alone or in combination with aspirin among pertinent patients.
Alongside this, recent studies [61, 62] have further questioned the clinical utility of measuring CYP2C19 endorsing our earlier comments that this measurement should not impact on the debate of whether to prescribe generic or originator clopidogrel.
There was already considerable variation in reimbursed prices for generic clopidogrel versus the originator, see Table 4, mirroring the findings in other studies [2, 3, 4, 6]. Again the size of the country’s population does not appear to be responsible for these differences, confirming previous publications [37]. Price reductions appear to be determined largely by ongoing policies to enhance generics utilisation [1–4, 52]. It is likely though that in time reimbursed prices for clopidogrel will converge, driven largely by countries striving to release further resources from the increasing availability of generics [53]. This will be researched in future studies alongside the impact of the various policies in each country to enhance the prescribing of generic clopidogrel versus the originator, see Table 2.
In conclusion, payers across Europe are learning from each other how best to take full advantage of the early availability of generics, even when there are different salts and indications, to maximise the use of available resources. This will continue. However, as we have seen this is not always possible. We believe pharmaceutical companies should accept generics availability to enable continued funding of new premium priced products, and not try to delay their introduction through challenging reimbursement decisions. The alternative, as resource pressures continue growing, is limited or no funding for new drugs, which is not in the future interests of all key stakeholder groups [1–4, 8, 54].
For patients
Pharmaceutical expenditure is typically the largest or equalling the largest component of expenditure in ambulatory, i.e. non-hospital, care. Consequently, the increasing availability of multiple sourced products (generics) once a product loses its patent is welcomed by health authorities and health insurance agencies as these can be provided at considerably lower costs than the originator. This is the case with generic clopidogrel with its price already only 12% of the cost of the originator within a few months in some European countries, with prices expected to fall further.
However, there can be concerns among physicians and patients with the effectiveness of a generic drug if this is provided as a different salt to the originator. The availability, and hence effectiveness of a generic drug, is tested though by the European authorities before such medications can become available to help address such fears. In this case, the European authorities found no bioavailability problems with different salts of generic clopidogrel compared to the originator substance. The European authorities go on testing generics to ensure trust in the system, and will remove generics if there are justified concerns. This happened with some of the manufacturers of generic clopidogrel giving further confidence in the system.
Health authorities and health insurance companies across Europe also typically found no issue with early formulations of generic clopidogrel despite different salts indications than the originator drug. Consequently, they typically took a pragmatic approach to encourage physicians to prescribe generic clopidogrel versus the originator to release considerable monies. Patients can also play their part by accepting generics that have been approved by the European authorities rather than the originators, with the monies released used to help maintain the European ideals of comprehensive and equitable health care in these difficult economic times especially in Europe.
Disclosure of financial interest
The majority of the authors are employed directly by health authorities or health insurance agencies or are advisers to these organisations. No author has any other relevant affiliation or financial involvement with any organisation or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript.
Acknowledgements
The study was in part supported by grants from the Karolinska Institutet and the Swedish Reimbursement Agency. We thank Ms Margaret Ewan from HAI Global for her help with reimbursed prices in New Zealand.
No writing assistance was utilised in the production of this manuscript.
Provenance and peer review: Commissioned; externally peer reviewed.
Authors
1Austrian Medicines and Medical Devices Agency, 9 Schnirchgasse, AT-1030 Wien, Austria
2 Institute for Pharmacological Research Mario Negri, 19 Via Giuseppe La Masa, IT-20156 Milan, Italy
3 Prescribing Research Group, University of Liverpool Management School, Chatham Street, Liverpool L69 7ZH, UK
4 Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186, Stockholm, Sweden
5 Department of Medicine Solna, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Solna, SE-17176, Stockholm, Sweden
6 Centre for Pharmacoepidemiology, Karolinska Institute, Karolinska University Hospital, Stockholm, Solna, Sweden
7 Drugs and Medical Products Regulation, Health Authority Abu Dhabi (HAAD), PO Box 5674, Abu Dhabi, United Arab Emirates
8 Strathclyde Institute for Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK
9 Information Services Division, NHS National Services Scotland, 1 South Gyle Crescent, Edinburgh EH12 9EB, UK
10 Hauptverband der Österreichischen Sozialversicherungsträger, 21 Kundmanngasse, AT-1031 Wien, Austria
11 Health Insurance Institute, 24 Miklosiceva, SI-1507 Ljubljana, Slovenia
12 Medicines Reimbursement Department, National Health Insurance Fund, 147 Kalvarijų Str, LT-08221 Vilnius, Lithuania
13 Kassenärztliche Vereinigung Hessen, 15 Georg Voigt Strasse, DE-60325 Frankfurt am Main, Germany
14 Republic Institute for Health Insurance, 2 Jovana Marinovica, 11000 Belgrade, Serbia
15 Research Department, The Social Insurance Institution, PO Box 450, FI-00101 Helsinki, Finland
16 State Agency of Medicines, 1 Nooruse, EE-50411 Tartu, Estonia
17 Medicines Management, NHS North Lancashire, Moor Lane Mills, Moor Lane, Lancaster LA1 1QD, UK
18 HTA Consulting, 17/3 Starowiślna Str, PL-31038 Cracow, Poland
19 Norwegian Medicines Agency, 8 Sven Oftedals vei, NO-0950 Oslo, Norway
20 University of Heidelberg, Institute of Pharmacology, DE-69120 Heidelberg, Germany
21 IRDES, 10 rue Vauvenargues, FR-75018 Paris, France
22 Dental and Pharmaceuticals Benefits Agency (TLV), PO Box 22520, 7 Flemingatan, SE-10422 Stockholm, Sweden
23 CEFAR – Center for Health Evaluation & Research, National Association of Pharmacies (ANF), 1 Rua Marechal Saldanha, PT-1249-069 Lisbon, Portugal
24 Instituut voor Verantwoord Medicijngebruik, Postbus 3089, 3502 GB Utrecht, The Netherlands
25 Quality Use of Medicines and Pharmacy Research Centre, Sansom Institute, School of Pharmacy and Medical Sciences, University of South Australia, Adelaide SA 5001, Australia
26 Ministry of Health, Republic of Croatia, Ksaver 200a, Zagreb, Croatia
27 Barcelona Health Region, Catalan Health Service, 30 Esteve Terrades, ES-08023 Barcelona, Spain
References
1. Godman B, Wettermark B, Bishop I, Burkhardt T, et al. European payer initiatives to reduce prescribing costs through use of generics. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(1):22-7. doi:10.5639/gabij.2012.0101.007
2. Godman B, Shrank W, Wettermark B, Andersen M, et al. Use of generics – a critical cost containment measure for all healthcare professionals in Europe? Pharmaceuticals. 2010;3:2470-94. doi:10.3390/ph/3082470 ISSN 1424-8247
3. Godman B, Shrank W, Andersen M, Berg C, Bishop I, et al. Comparing policies to enhance prescribing efficiency in Europe through increasing generic utilisation: changes seen and global implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10:707-22.
4. Godman B, Shrank W, Andersen M, Berg C, Bishop I, et al. Policies to enhance prescribing efficiency in Europe: findings and future implications. Front Pharmacol. 2011;1(141):1-16. doi:10.3389/fphar.2010.00141
5. Garattini S, Bertele V, Godman B, Haycox A, et al. Enhancing the rational use of new medicines across European healthcare systems – A position paper. Eur J Clin Pharmacol. 2008;64(12):1137-8.
6. Godman B, Sakshaug S, Berg C, Wettermark B, Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev Pharmacoecon Outcomes Res. 2011;11:121-9.
7. Von ina L, Strizrep T, Godman B, Bennie M, et al. Influence of demand-side measures to enhance renin-angiotensin prescribing efficiency in Europe: implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2011; 11:469-79.
8. Godman B, Wettermark B, Bennie M, Burkhardt T, Garuoliene K, et al. Enhancing prescribing efficiency through increased utilisation of generics at low prices. (E) Hospital 2011;13(3):28-31. Available from: myhospital.eu/journals/articles/enhanc-ing_prescribing_efficiency_through_increased_utilisation_generics_low_prices
9. Pharma Live [homepage on the Internet]. Top 500 prescription medicines. Sales Plavix 2009. [cited 2012 May 22]. Available from: www.pharmalive.com/special_reports/sample.cfm?reportID=314
10. GaBI Online – Generics and Biosimilars Initiative. 2012’s biggest patent expiries [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 May 22]. Available from: www.gabionline.net/Policies-Legislation/2012-s-biggest-patent-expiries
11. IHS Global Insight [homepage on the Internet]. Sanofi-Aventis faces new round of generic threats to plavix in Europe. [cited 2012 May 22]. Available from: www.ihsglobalinsight.com/SDA/SDADetail16688.htm
12. Shuchman M. Delaying generic competition – corporate payoffs and the future of Plavix. N Engl J Med. 2006;355:1297-300.
13. Acino [homepage on the Internet]. EMEA-Committee recommends approval of Acino’s generic clopidogrel in Europe. [cited 2012 May 22]. Available from: www.acino-pharma.com/html/uploads/media/Acino_Clopi_PressRel_E_090601.pdf
14. Lisa Nainggolan. Six generic clopidogrel versions pass first EU marketing hurdle. June 2009 [cited 2012 May 22]. Available from: www.theheart.org/article/975593.do
15. PSNC [homepage on the Internet]. When generic clopidogrel available and dispensing based on salts. [cited 2012 May 22]. Available from: www.psnc.org.uk/news.php/610/clopidogrel_75mg_tablets_to_move_to_part_viii_category_a_in_December
16. NHS Greater Glasgow and Clyde [homepage on the Internet]. Postscript primary care. 2010 [cited 2012 May 22]. Available from: www.glasgowformu-lary.scot.nhs.uk/prescriber/PSPCFebruary2010.pdf
17. Pereillo JM, Maftouh M, Andrieu A, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos. 2002;30:1288-95.
18 Davies G. Changing the salt, changing the drug. Pharm J. 2001;266:322-3.
19. Takahashi M, Pang H, Kawabata K, et al. Quantitative determination of clopidogrel active metabolite in human plasma. J Pharm Biomed Anal. 2008;48:1219-24.
20. Pawlowska M, Duda J, Tejchman-Malecka B, et al. Usefulness of the parent compound determination in bioequivalence evaluation of clopidogrel generic products. Arzneimittelforschung. 2009;59:289-96.
21. Rocca B, Patrono C. Determinants of the interindividual variability in response to antiplatelet drugs. J Thromb Haemost. 2005;3:1597-602.
22. Baumgärtel C, Garuoliene K, Godman B, Skiöld P, Bishop I, Burkhardt T, et al. Enhancing the utilisation of generic clopidogrel: a case history for future guidance? Proceedings of the PPRI Conference 2011; 29–30 September 2011; Vienna, Austria; WHO Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policies at the Austrian Health Institute (Gesundheit Österreich Gmbh); 2011. [cited 2012 May 22]. Available from: whocc.goeg.at/Downloads/Conference2011/PraesentationenPPRIKonferenz/general_PPRI%20conference%202011%20Abstract%20book.pdf
23. Epstein R, Russell Teagarden J. Comparative effectiveness research and personalized medicine catalyzing or colliding? Pharmacoeconomics. 2010;28(10):905-13.
24. Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244-7.
25. Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009 Jan 24;373(9660):309-17.
26. Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008 May 20;51(20):1925-34.
27. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al.; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001-15. Epub 2007 Nov 4.
28. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009 Jan 22;360(4):354-62.
29. Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al.; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009 Jan 22;360(4):363-75. Epub 2008 Dec 22.
30. Sheppard A. Generic medicines: essential contributors to the long-term health of society. Sector sustainability challenges in Europe [homepage on the Internet]. [cited 2012 May 22]. Available from: www.imshealth.com/imshealth/Global/Content/Document/Market_Measurement_TL/Generic_Medicines_GA.pdf
31. European Medicines Agency [homepage on the Internet]. Temporary withdrawal generic clopidogrel 2010. [cited 2012 May 22]. Available from: www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/03/WC500076546.pdf
32. European Medicines Agency [homepage on the Internet]. EMA inspections. [cited 2012 May 22]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001054/WC500102581.pdf
33. Inpharm [homepage on the Internet]. Withdrawal generic clopidogrel. 2010 March. [cited 2012 May 22]. Available from: www.inpharm.com/news/ema-calls-generic-clopidogrel-recall
34. GaBI Online – Generics and Biosimilars Initiative. EMA wants recall of Acino’s generic clopidogrel with active substance of Indian Glochem [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 May 22]. Available from: www.gabionline.net/Generics/News/EMA-wants-recall-of-Acino-s-generic-clopidogrel-with-active-substance-of-Indian-Glochem
35. Sanofi-aventis [homepage on the Internet]. Sanofi-aventis Confirms Quality and Supply of Plavix®, Iscover® and Clopidogrel Winthrop® Paris. March 2010. [cited 2012 May 22]. Available from: en.sanofi-aventis.com/binaries/20100326_Clopidogrel_en_tcm28-27815.pdf
36. Godman B, Abuelkhair M, Vitry A, Abdu S, et al. Payers endorse generics to enhance prescribing efficiency; impact and future implications, a case history approach. Generics and Biosimilars Initiative Journal (GaBI Journal). 2012;1(2):69-83. doi:10.5639/gabij.2012.0102.017
37. Garuoliene K, Godman, B, Gulbinovič J, et al. European countries with small populations can obtain low prices for generics: Lithuania as a case history. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):343-9.
38. McGinn D, Godman B, Lonsdale J, et al. Initiatives to enhance the quality and efficiency of statin and PPI prescribing in the UK: impact and implications. Expert Rev Pharmacoecon Outcomes Res. 2010;10(1):73-85.
39. Godman B, Burkhardt T, Bucsics A, et al. Impact of recent reforms in Austria on utilisation and expenditure of PPIs and lipid lowering drugs; implications for the future. Expert Rev Pharmacoecon Outcomes Res. 2009;9:475-84.
40. Coma A, Zara C, Godman B, Augusti A, Diogene E, et al. Policies to enhance the efficiency of prescribing in the Spanish Catalan region: impact and future direction. Expert Rev Pharmacoecon Outcomes Res. 2009;9:569-81.
41. Godman B, Wettermark B, Hoffman M, et al. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden; global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9:65-83.
42. Abuelkhair M, Abdu S, Godman B, et al. Imperative to consider multiple initiatives to maximise prescribing efficiency from generic availability: case history from Abu Dhabi. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):115-24.
43. Sturm H, Austvoll-Dahlgren A, Aaserud M, et al. Pharmaceutical policies: effects of financial incentives for prescribers. Cochrane Database Syst Rev. 2007(3): Art No.: CD006731. doi:10.1002/14651858.CD006731
44. World Health Organization [homepage on the Internet]. Introduction to drug utilisation research. WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. ISBN 92 4 156234 X (NLM classification: WB 330). 2003 [cited 2012 May 22]. Available from: www.who.int/medicines/areas/quality_safety/safety_efficacy/Drug%20utilization%20research.pdf
45. Wettermark B, Godman B, Jacobsson B, Haaijer-Ruskamp F. Soft regulations in pharmaceutical policymaking – an overview of current approaches and their consequences. Appl Health Econ Health Policy. 2009;7:137-47.
46. Gustafsson LL, Wettermark B, Godman B, Andersén-Karlsson E, Bergman U, et al. The ‘Wise List’ – a comprehensive concept to select, communicate and achieve adherence to recommendations of essential drugs in ambulatory care in Stockholm. Basic Clin Pharmacol Toxicol. 2011;108(4):224-33.
47. Voncina L, Strizrep T. Croatia: 2009/2010 pharmaceutical pricing and reimbursement forum. Eurohealth. 2011;16:20-2.
48. GaBI Online – Generics and Biosimilars Initiative. Sanofi-aventis still owns clopidogrel users patent for acute coronary syndrome, in contrast to most generic clopidogrels [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 May 22] Available from: www.gabionline.net/Generics/News/Sanofi-aventis-still-owns-clopidogrel-users-patent-for-acute-coronary-syndrome-in-contrast-to-most-generic-clopidogrels
49. Generic clopidogrel an update from HSC [homepage on the Internet]. March 2011. [cited 2012 May 22]. Available from: www.hscboard.hscni.net/medi-cinesmanagement/Prescribing%20Guidance/033%20Guidance%20on%20Clopidogrel%20-%20An%20Update%20for%20HSC%20March%202011%20-%20PDF%20221KB.pdf
50. Wettermark B, Godman B, Andersson K, et al. Recent national and regional drug reforms in Sweden – implications for pharmaceutical companies in Europe. Pharmacoeconomics. 2008;26:537-50.
51. Cramer JA, Benedict A, Muszabek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62:76-87.
52. Dylst P, Simoens S. Does the market share of generic medicines influence the price level? A European analysis. Pharmacoeconomics. 2011 Oct;29(10):875-82. doi:10.2165/11585970-000000000-00000
53. Jack A. Balancing big pharma’s books. BMJ. 2008;336:418-9.
54. Godman B, Malmström RE, Bennie M, Sakshaug S, Burkhardt T, et al. Prescribing restrictions – a necessary strategy among some European countries to enhance future prescribing efficiency? Reviews in Health Care. 2012;3(1):5-16.
55. Baumgärtel C. Clopidogrel generika sind gleichwertig. Therapie info. 2010; 12(3):3-6. [cited 2012 May 22]. Available from: www.sozialversicherung.at/mediaDB/692427_therapieinfo_nr3_07_2010.pdf
56. Collet JP, et al. Antiagrégants plaquettaires oraux en pratique quotidienne. La lettre du cardiologue. 2010;431:16-22.
57. Bagheru H, Montastruc Jean-Louis, O Lavezzi. Plavix (clopidogrel) et ses-generiques: parfait example de confusion reglementaire, au profit de qui? Prescrire. July 2010;30(321):353. [cited 2012 May 22]. Available from: www.chu-toulouse.fr/IMG/pdf/plavix_generiques_prescrire_juillet_2010-2.pdf
58. Autorite de la concurrence. Décision n° 10-D-16 du 17 mai 2010 relative à des pratiques mises en oeuvre par la société Sanofi-Aventis France. [cited 2012 May 22]. Available from: www.autoritedelaconcurrence.fr/pdf/avis/10d16.pdf
59. Sermet C, Andrieu V, Godman B, Van Ganse E, Haycox A, Reynier JP. Ongoing pharmaceutical reforms in France; implications for key stakeholder groups. App Health Econ Health Policy. 2010;8(1):7-24.
60. Garuoliene K, Alonderis T, Marcinkevi ius M. Pharmaceutical policy and the effects of the economic crisis: Lithuania. Eurohealth. 2011;17:1-4.
61. Holmes M, Perel P, Shah T, Hingorani A, Casas J. CYP2C19 genotype, clopi-dogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704-14.
62. Wang L, McLeod H, Weinshilboum R. Genomics and drug response. NEJM. 2011;364:1144-53.
|
Author for correspondence: Brian Godman, BSc, PhD, Department of Laboratory Medicine, Division of Clinical Pharmacology, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-14186, Stockholm, Sweden |
Disclosure of Conflict of Interest Statement is available upon request.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Related article
Generic clopidogrel–the medicines agency’s perspective



This is my first time go to see at here and I am truly pleassant to read all at alone
place.
Dear Mr Sean McKeon,
We very much appreciate your kind feedback.
Thank you for your interest in GaBI. Please enjoy the quality information and content published under GaBI (GaBI Online and GaBI Journal).
GaBI Journal Editorial Office