Current trends in biosimilar uptake and special focus on automatic substitution – a symposium report
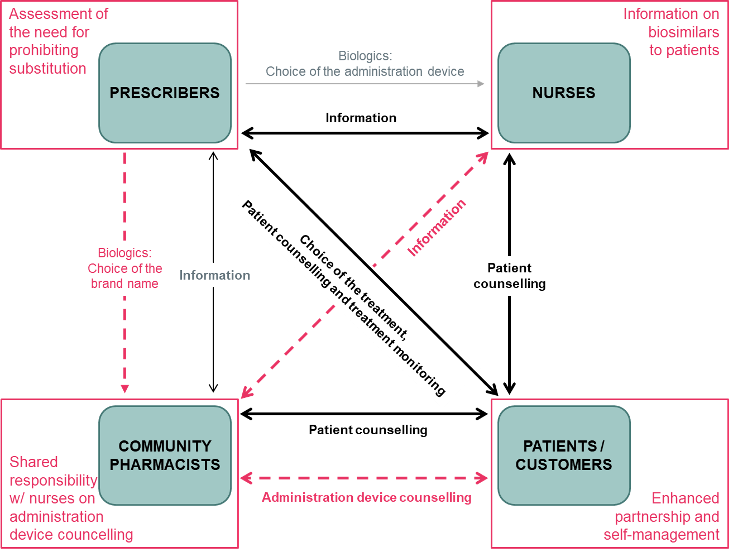
Abstract: In December 2023, the University of Helsinki, Helsinki University Hospital (HUS), and University Pharmacy (Yliopiston Apteekki) Helsinki collaborated to present the online symposium titled ‘Current Trends in Biosimilar Uptake and Research with Special Focus on Automatic Substitution’. This provided an overview of global trends in biosimilar use and a systematic examination of the implications […]




