 Original Research
Original Research
Published on 28 November 2023
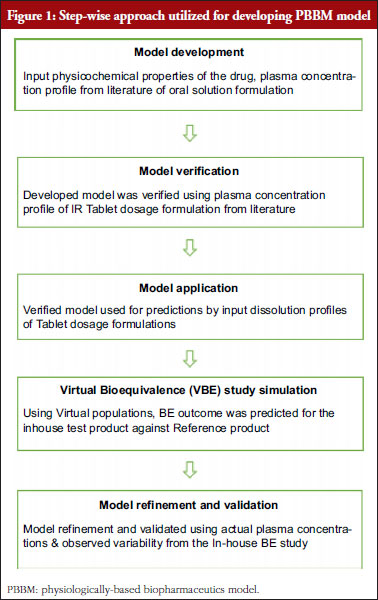
Pharmacokinetic bioequivalence studies of a new Etoricoxib tablet formulation developed using proprietary MiST technology — risk assessment and mitigation using GastroPlus software
etoricoxib, GastroPlus®, modelling and simulation, PBBM, spray granulation, virtual bioequivalence
DOI: 10.5639/gabij.2024.1301.003
3.914 views
 Editor's Letter
Editor's Letter
 Editor's Letter
Editor's Letter
 Editor's Letter
Editor's Letter
 Editor's Letter
Editor's Letter
 Sponsored Article
Sponsored Article


