Published on 07 May 2024
Increasing adoption of quality-assured biosimilars to address access challenges in low- and middle-income countries
affordability, biosimilars, low- and middle-income countries (LMICs), market access, non-communicable diseases, regulatory framework
DOI: 10.5639/gabij.2024.1302.008
6.558 views
 Table Contents
Table Contents
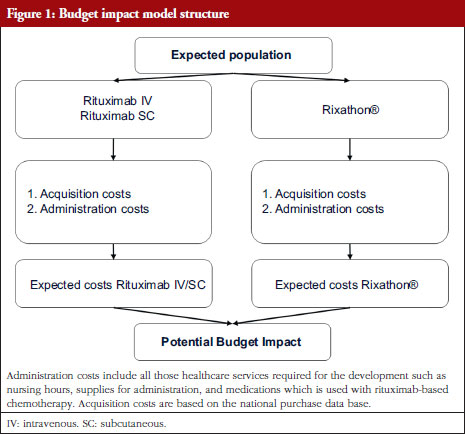 Original Research
Original Research
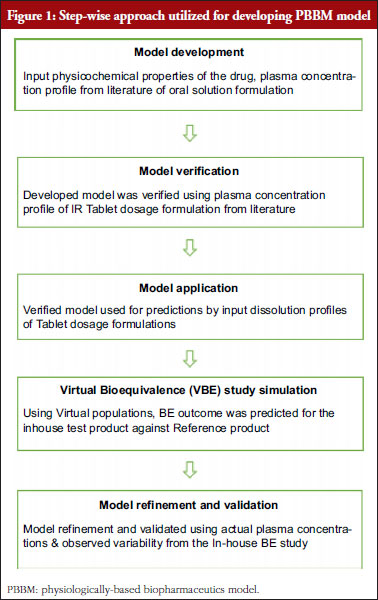 Original Research
Original Research
 Editor's Letter
Editor's Letter
 Editor's Letter
Editor's Letter
 Meeting Report
Meeting Report


